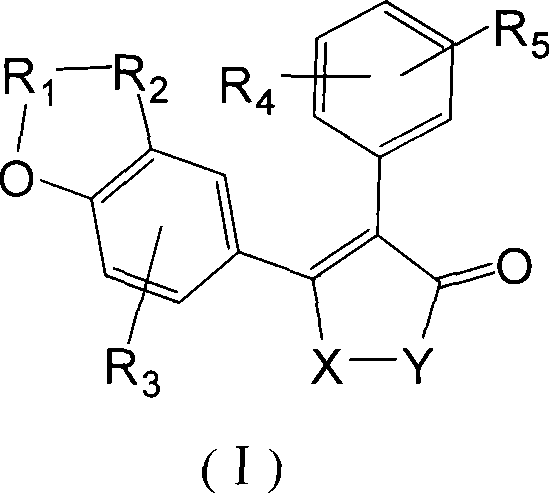
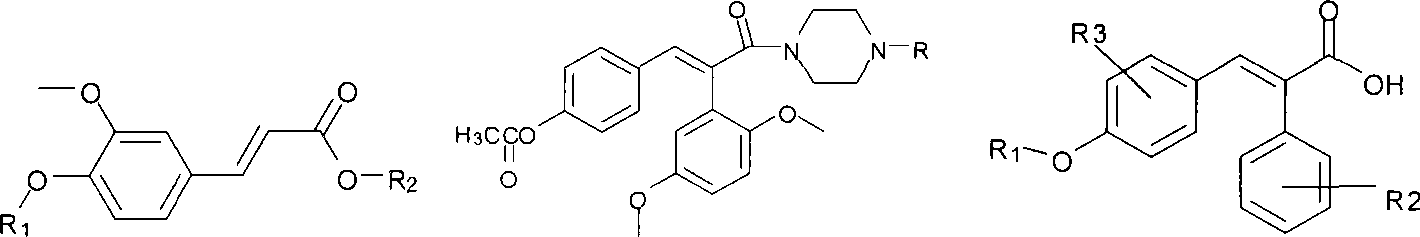
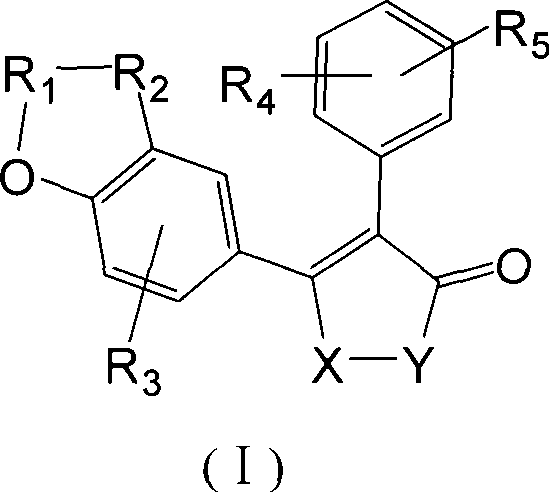
P-hydroxybenzene acrylic acid derivative and uses thereof
A technology of hydroxyphenylacrylic acid and derivatives, which is applied in the direction of drug combinations, medical preparations containing active ingredients, organic active ingredients, etc., and can solve problems such as osteoarthritis and rheumatoid arthritis without giving up
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0168] Embodiment 1: Preparation of (E)-2-(4-sulfonamidophenyl)-3-(3-methoxy-4-acetoxyphenyl)acrylic acid
[0169]
[0170] In a four-neck flask, add 0.25g (0.0013mol) of acetylvanillin to 10mL of acetonitrile solvent, add 0.4mL of triethylamine, stir to dissolve, then add 0.22g (0.001mol) of p-sulfonamide phenylacetic acid, and stir to react After 1 hour, DBU was added, the reaction was stirred for another 1 hour, and the reaction was stopped. About 10 mL of 1N hydrochloric acid was added, extracted with ethyl acetate, and the layers were separated. The ethyl acetate solution was washed with saturated brine, dried, and concentrated to obtain a yellow solid. Weight 0.06g. Thin layer, Rf is 0.3.
[0171] 1 H-NMR (DMSO) δ (ppm): 1.94 (3H, s, COCH 3 ), 3.72 (3H, s, OCH 3 ), 7.33 (2H, NH 2 ), 7.14-7.80 (7H, m, hydrogen on the benzene ring), 7.58 (1H, -C=C-H), 12.36 (1H, s, COOH);
[0172] MS(m / z): 392.3[M+H] + , 409.4[M+NH 4 ] + ;
[0173] IR (KBr) (v / cm-1): 3434, 323...
Embodiment 2
[0174] Example 2: Preparation of 3(4-sulfonamidophenyl)-4-(3-methoxy 4-hydroxyphenyl)-2(5H)-furanone
[0175]
[0176] In a four-necked flask, add 0.37 g (0.0015 mol) of 3-methoxy 4-hydroxybromoacetophenone into 10 mL of acetonitrile solvent, add 0.4 mL of triethylamine, and then add 0.22 g of p-sulfonamidophenylacetic acid (0.001mol), stirred and reacted for 1 hour, then added DBU, stirred and reacted for 2 hours, stopped the reaction, added about 10 mL of 1N hydrochloric acid, extracted with ethyl acetate, separated layers, washed the ethyl acetate solution with water, dried, and concentrated to obtain yellow solid. Thin layer, Rf is 0.4.
[0177] 1 H-NMR (DMSO) δ (ppm): 5.44 (2H, s, CH 2 ), 7.24-7.73 (7H, m, benzene ring hydrogen), 7.45 (2H, NH 2 );
[0178] MS(m / z): 362.2[M+H] + ;
[0179] IR (KBr) (v / cm-1): 3431, 3230 (NH 2 ); 3010 (benzene ring hydrogen); 2940 (OCH 3 ); 1740 (furanone); 1192, 1141 (SO 2 ).
Embodiment 3
[0180] Embodiment 3: Preparation of (E)-2-(4-sulfonamidophenyl)-3-(3-methoxyl-4-hydroxylphenyl)acrylic acid
[0181]
[0182] In a four-neck flask, add 0.23g (0.0015mol) of vanillin to 10mL of acetonitrile solvent, add 0.40mL of triethylamine, stir to dissolve, then add 0.22g (0.001mol) of p-sulfonamide phenylacetic acid, and stir to react 1 After 2 hours, DBU was added, and the reaction was stirred for another 2 hours. The reaction was stopped, and about 10 mL of 1N hydrochloric acid was added, extracted with ethyl acetate, and the layers were separated. The ethyl acetate solution was washed with water, dried, and concentrated to obtain a yellow solid. Thin layer, Rf is 0.15.
[0183] MS(m / z): 350.3[M+H] + , 367.4[M+NH 4 ] + ;
[0184] IR (KBr) (v / cm-1): 3433, 3230 (NH 2 , COOH); 3020, 2977 (benzene ring hydrogen); 2942 (OCH 3 ); 1677(υ C=0 ,—CH=CCOOH); 1190,1154 (SO 2 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com