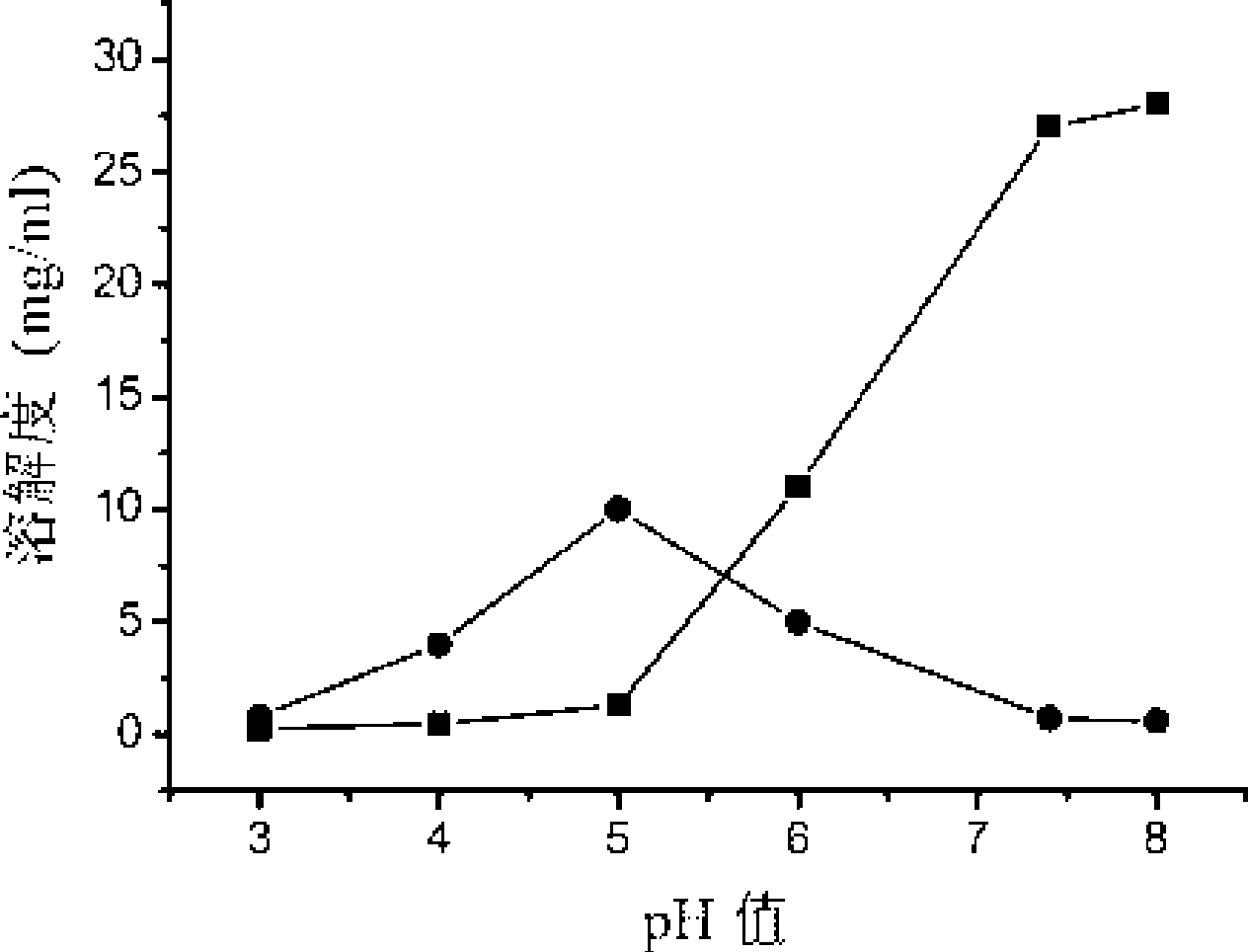
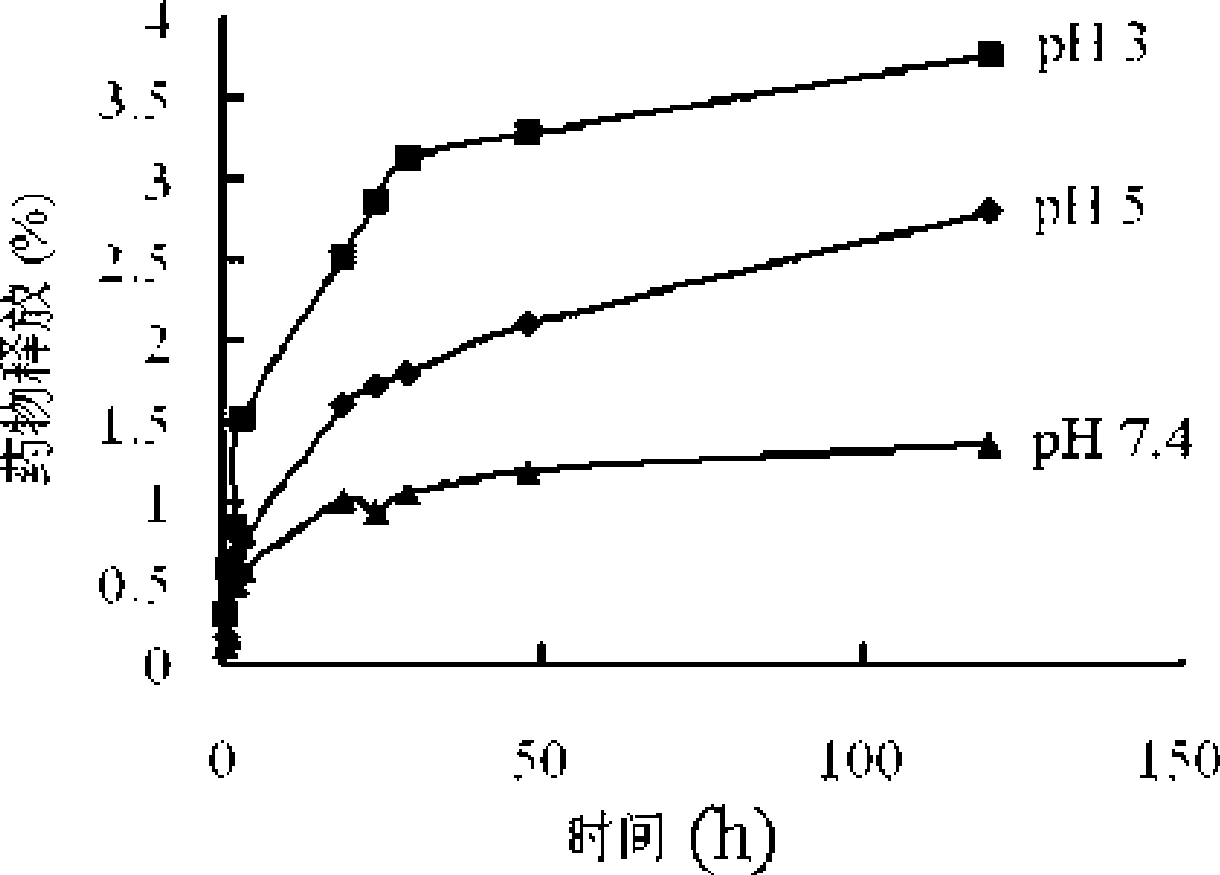
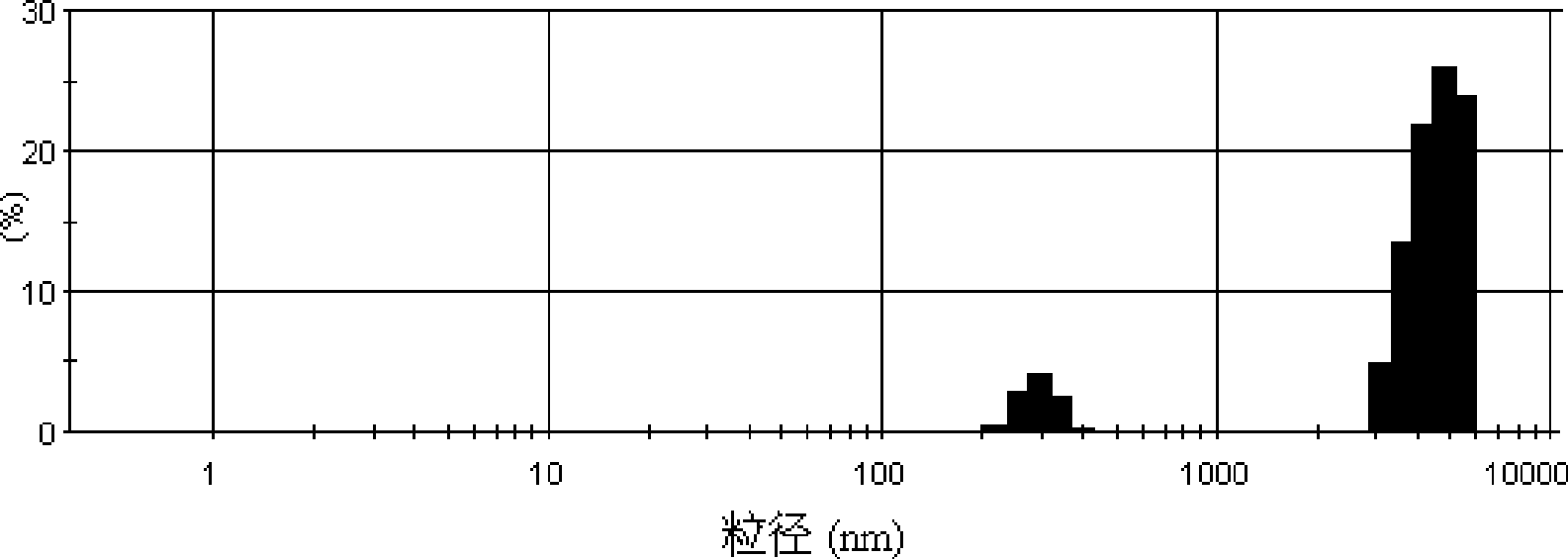
PH sensitivity type anticancer prodrug as well as preparation method and application thereof
An anti-cancer drug and sensitive technology, applied in the field of medicine and chemical industry, can solve the problems of reducing the therapeutic effect of doxorubicin, reducing the solubility, toxic and side effects, etc., and achieving the effect of good phagocytosis, small particle size and narrow distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1 Preparation of pectin-doxorubicin conjugate of the present invention
[0029] Weigh 1g of pectin (molecular weight 71,000-400,000, average molecular weight 200,000), add 100ml of water, after the pectin is dissolved, adjust the pH to 6-8 with sodium hydroxide solution. Dissolve 0.5 g of doxorubicin hydrochloride in 100 ml of water, transfer to the pectin solution, control the temperature at 50° C., and stir for 30 minutes. 1 g of EDC·HCl was added, the temperature was controlled at 50° C., and the reaction was stirred for 6.5 hours. After reaching the required time, it was transferred to a dialysis bag with a molecular weight cut off of 7000, dialyzed with secondary water for 24 hours, and the dialyzate was concentrated to dryness to obtain 0.9 g of a red solid.
Embodiment 2
[0030] Embodiment 2 Preparation of pectin-doxorubicin conjugate of the present invention
[0031] Weigh 1g of pectin (molecular weight 71,000-400,000, average molecular weight 200,000), add 100ml of water, after the pectin is dissolved, adjust the pH to 6-8 with sodium hydroxide solution. Dissolve 0.5 g of doxorubicin hydrochloride in 100 ml of water, transfer to the pectin solution, control the temperature at 50° C., and stir for 30 minutes. 1 g of EDC·HCl was added, the temperature was controlled at 50° C., and the reaction was stirred for 8 hours. After reaching the required time, it was transferred to a dialysis bag with a molecular weight cut off of 7000, dialyzed with secondary water for 24 hours, and the dialyzate was concentrated to dryness to obtain 1.1 g of a red solid.
Embodiment 3
[0032] Example 3 Preparation of Pectin-Adriamycin Conjugate of the Present Invention
[0033] Weigh 1g of pectin (molecular weight 71,000-400,000, average molecular weight 200,000), add 100ml of water, after the pectin is dissolved, adjust the pH to 6-8 with sodium hydroxide solution. Dissolve 0.5 g of doxorubicin hydrochloride in 100 ml of water, transfer to the pectin solution, control the temperature at 30° C., and stir for 30 minutes. 1 g of EDC·HCl was added, the temperature was controlled at 30°C, and the reaction was stirred for 24 hours. After reaching the required time, it was transferred to a dialysis bag with a molecular weight cut off of 7000, dialyzed with secondary water for 24 hours, and the dialyzate was concentrated to dryness to obtain 1 g of a red solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com