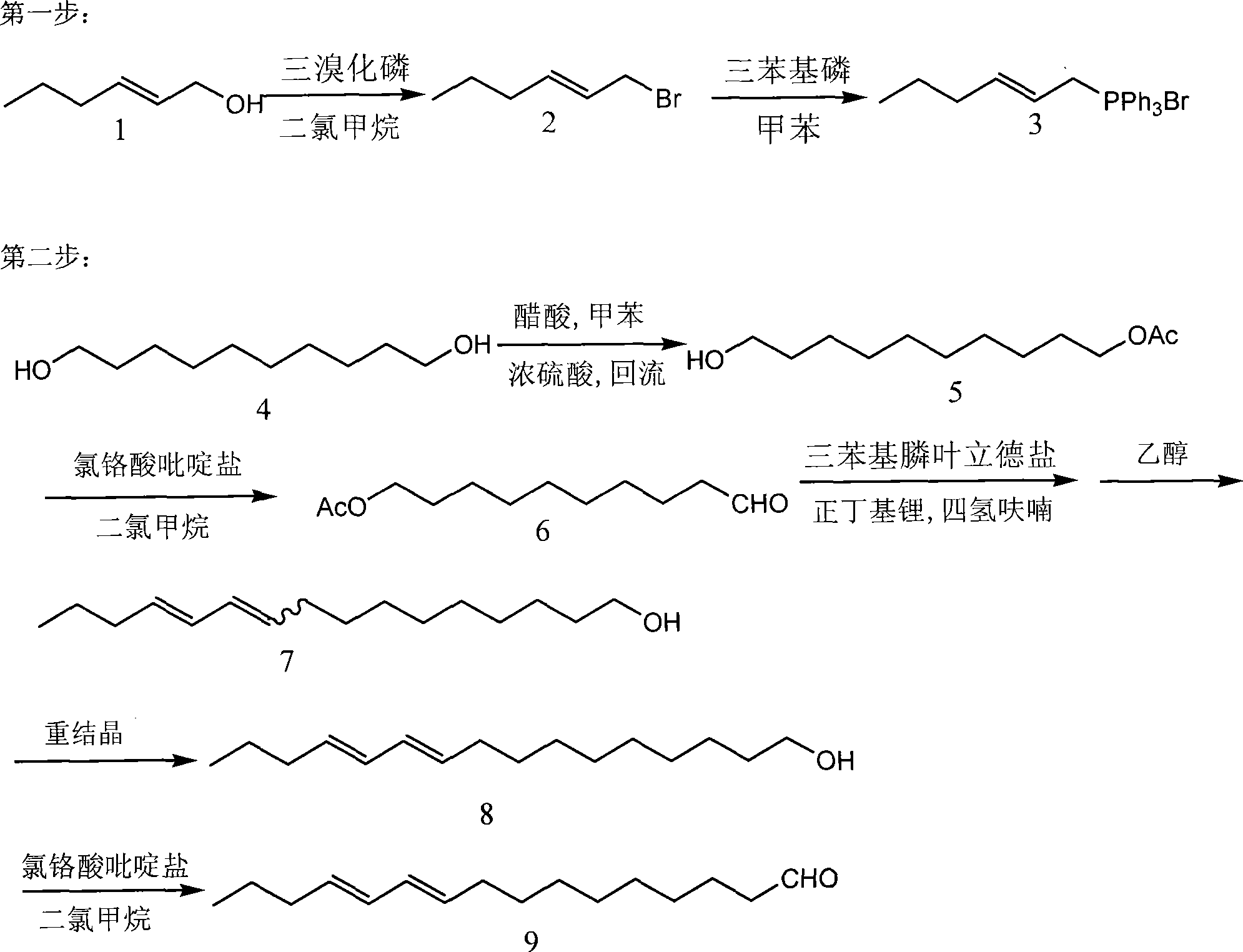
Method for synthesizing compound E10, E12-hexadecadienal in sex pheromone of legume pod borer
A synthesis method and a technology for the properties of Pseudomonas spp. are applied to compound E10 in the sex pheromone of Pseudomonas spp., which can solve the problems of large residues, excessive pesticide residues, short harvest time and the like, and achieves simple and safe operation, high utilization rate of raw materials, Simple to use effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0024] Below in conjunction with embodiment the present invention is described in further detail.
[0025] 1. The preparation of compound (3) triphenylphosphine ylide salt: compound (1) trans-2-hexenol was dissolved in dry dichloromethane, cooled to-10°C under nitrogen protection, and slowly added dropwise 0.33% ( Mole percent) of phosphorus tribromide, after the dropwise addition, continue to stir for 30 minutes, quench the reaction with ice saturated sodium bicarbonate solution, add a large amount of petroleum ether to dilute, such as 5 or 10 times more, and then wash with saturated saline , then collect the organic phase and dry it with anhydrous sodium sulfate for half an hour, remove sodium sulfate by filtration, and remove sherwood oil with a rotary evaporator to obtain the crude product of compound (2) trans-1-bromo-2-hexene, Dissolve it in dry toluene without further purification, add 1.2% (mole percent) triphenylphosphine, stir in the dark for two days, and filter to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com