Novel flavonoid extracted from Maackia amurensis
A technology of flavonoids and compounds, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0008] Embodiment 1: the preparation of compound 1-7
[0009] 1. Extraction and separation
[0010] Take 9 kg of dried pagoda tree bark, heat and reflux extract with 70% ethanol for 4 times, each time for 3 hours, dry under reduced pressure, concentrate to obtain 1800 g of extract. The medicinal extract is suspended in water, and in a ratio of 1:1, it is extracted successively with petroleum ether, ethyl acetate, and n-butanol, and each solvent is extracted 3 times, and the extracted parts are respectively concentrated under reduced pressure to obtain 120 g of petroleum ether extracted parts. Carry out silica gel column chromatography (800g, 200-300 mesh), use petroleum ether-dichloromethane (10:1→1:1), dichloromethane-ethyl acetate gradient elution (50:1→1:1), A total of 109 fractions were collected, each with a volume of about 500 mL, and combined according to thin-layer chromatography to obtain 9 sub-fractions (Fr.1-Fr.9). Fr.3 by Sephadex LH-20 gel chromatography, CH 2 ...
Embodiment 2
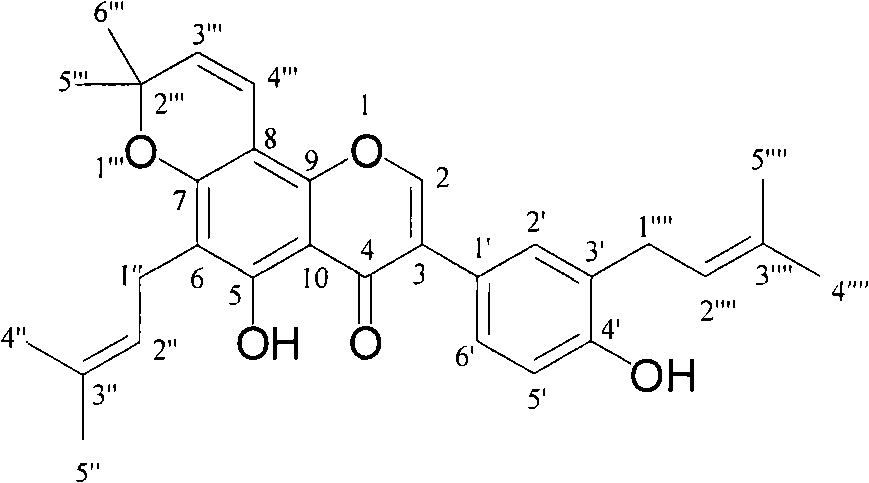
[0028] Example 2: Confirmation of the structure of compound 1-7
[0029] Table 1. Physicochemical constants and main spectral data of compounds 1-7
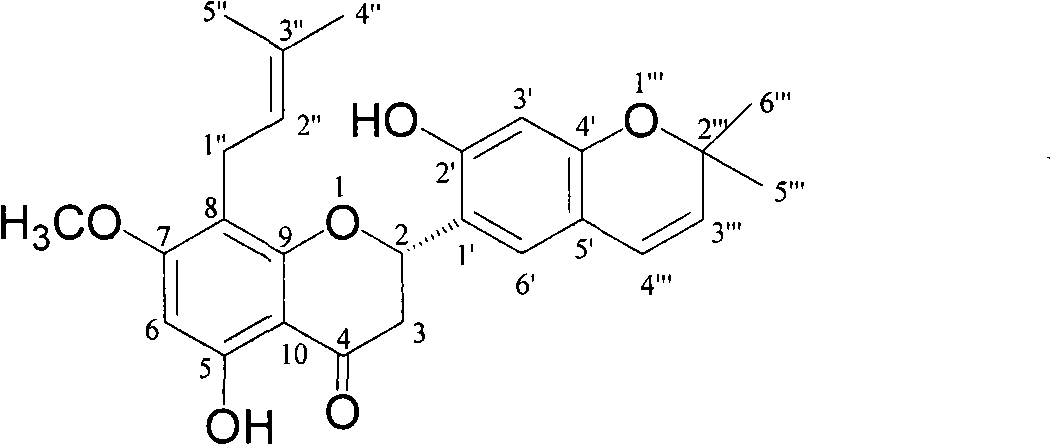
[0030] Compound 1
[0031] Molecular formula C 26 h 28 o 6 , It is a white amorphous powder. [α] 20 D =-67.3° (c, 0.1, MeOH), CD (c 0.05, MeOH; nm): [θ] 350 +0.92, [θ] 290 -6.20. UV(MeOH) has maximum absorption at 289nm. IR(KBr)υ max , cm -1 3443 (broad), 2925, 1634, 1502, 1445, 1383, 1206, 1167, 1106, 1073. ESI-MS m / z: 437.3[M+1] + , 435.3[M-1] - , 873.8[2M+1] + , 871.6[2M-1] - , HR-ESI-MS m / z 437.1968[M+H] + . 1 HNMR (600MHz, acetone-d 6 ) see Table 2. 13 CNMR (150MHz, acetone-d 6 ) see Table 2.
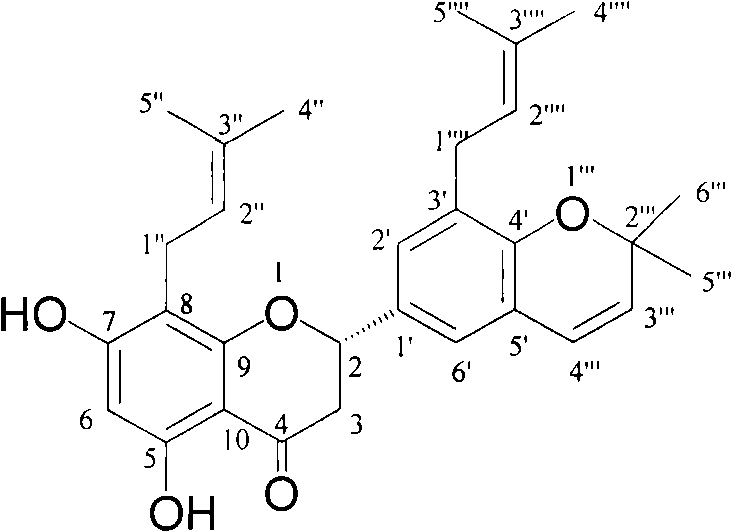
[0032] Compound 2
[0033] Molecular formula: C 30 h 34 o 5 , Pale yellow jelly. [α] 20 D = -34.7° (c, 0.2, MeOH). UV(MeOH) has maximum absorption at 293nm. IR(KBr)υ max cm -13420, 2973, 2923, 1637, 1505, 1435, 1383, 1170, 1148, 1077. ESI-MS m / z: 475.4[M+1] + , 473.4[M-1] - , HR-ESI-MS m / z 475.248...
Embodiment 3
[0052] Example 3: Antitumor activity of compounds 1-7
[0053] The in vitro antitumor activity of the above compounds was investigated by MTT method. The specific implementation method is as follows:
[0054] Human cervical cancer HeLa cells, human melanoma A375S2 cells, human breast cancer MCF-7 cells, and human liver cancer HepG2 cells were seeded in 96-well plates at a density of 1×105 / well, and after 12 hours of culture, samples of different concentrations were added to continue the culture. After 48 hours, the culture solution was discarded, and 100 μl of culture solution and 10 μl of 5 mg / ml MTT were added to each well, and cultured for another 4 hours, and the absorbance was measured with an enzyme-linked immunoassay analyzer. At the same time, a blank control and a negative control were set, and 5-fluorouracil was used as a positive control, and the mortality rate was calculated according to the following formula:
[0055]
[0056] The IC of each compound was calc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com