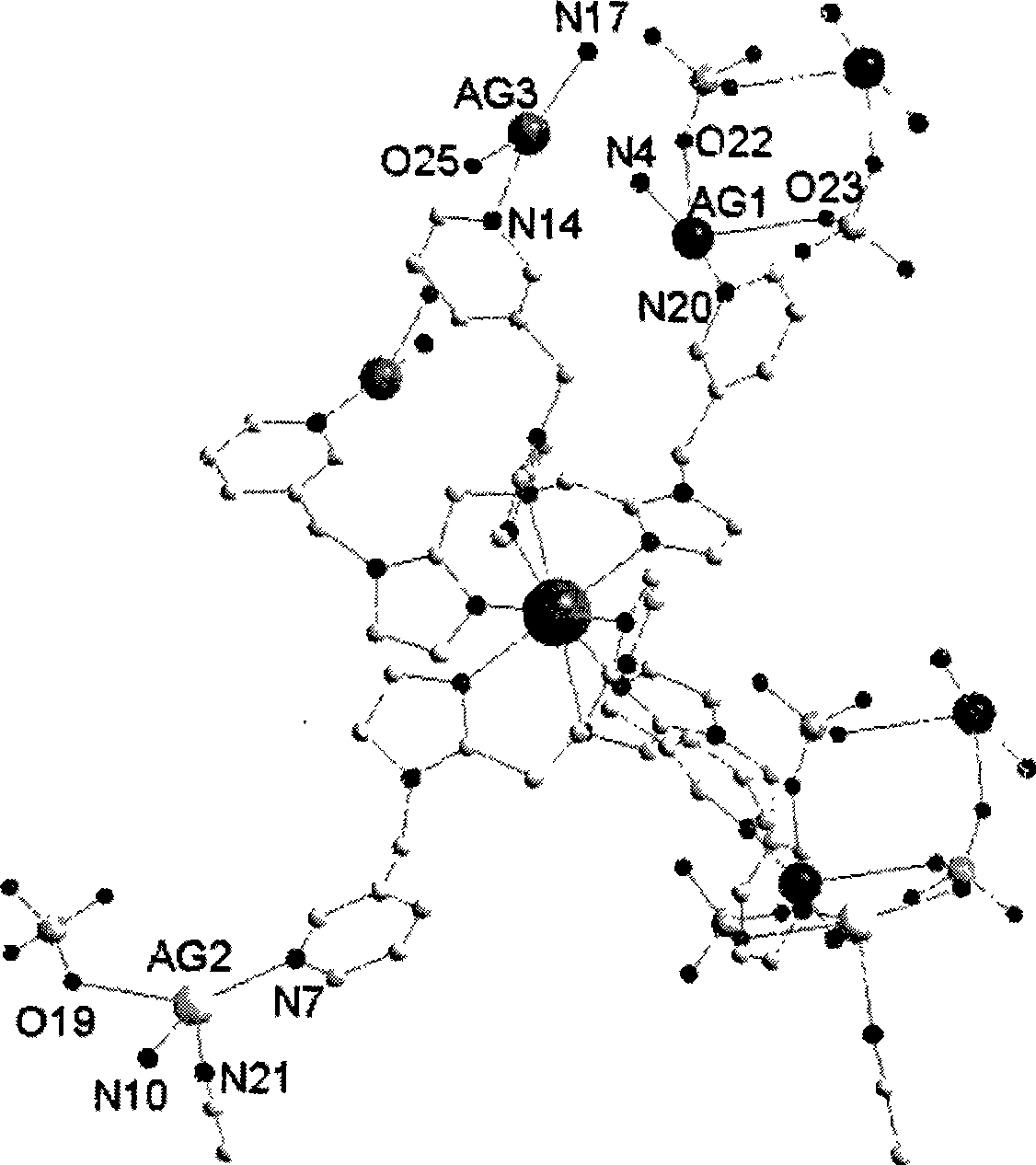
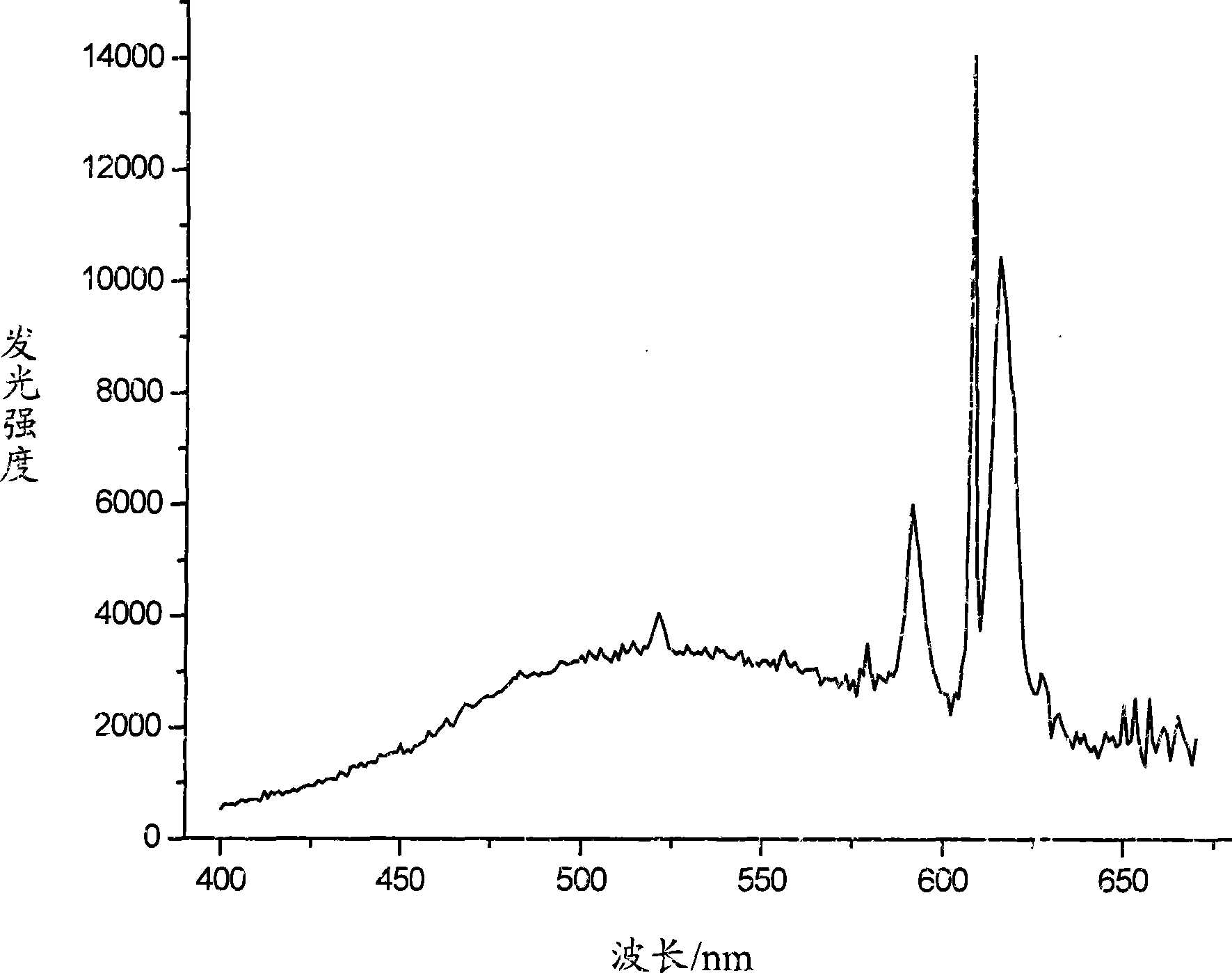
Mixed metal complex emitting white light and preparation thereof
A technology of mixing metals and complexes, which is applied in the direction of luminescent materials, compounds containing elements of group 3/13 of the periodic table, silver organic compounds, etc., and can solve problems such as brightness, efficiency, stability, and low reproducibility of performance parameters , to achieve the effect of improving luminous brightness, high luminous brightness, high stability and reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1 Preparation of Ligand I:
[0021] (1) Add 16.2g (0.15mol) of o-phenylenediamine and 9.6g (0.05mol) of amine triacetic acid into a 250ml round bottom flask, add 60ml of ethylene glycol and stir evenly, heat the oil bath to 140-200 degrees to reflux 8-12 hours, during the reaction process, a water separator can be installed to draw the generated water out of the reaction system; after the reaction, cool slightly while stirring and pour into 200ml of water, filter, recrystallize ethanol (decolorize with activated carbon if necessary), and obtain the product NTB (Yield 85%).
[0022] (2) In a 100ml flask, add 50% NaOH (25mmol) aqueous solution, 2.5mmol NTB, 20mg tetrabutylammonium bromide, and 40ml butanone, stir, heat to reflux, and add 8.75mmol chloromethyl 2 after the solution becomes clear. -Pyridine hydrochloride, continue to reflux for 5h, stop the reaction, pour the solution into 300ml water, wait overnight for butanone to volatilize, then filter, dissolve...
Embodiment 2
[0026] Preparation of Ligand II:
[0027] With step (1) (2) among the embodiment 1, but the chloromethyl 2-pyridine hydrochloride in (2) is changed into chloromethyl 3-pyridine hydrochloride.
[0028] NMR data of the resulting product:
[0029] 1 H-NMR (300MHz in DMSO-d 6 , 80°C): δ4.32 (6H, s, 1), 5.32 (6H, s, 6), 7.04 (3H, m, 4), 7.14 (9H, m, 2, 3, 5), 7.32 (3H , m, 9), 7.48 (3H, m, 10), 8.17 (3H, s, 7), 8.32 (3H, d, 8).
Embodiment 3
[0031] Preparation of Ligand III:
[0032] Same as step (1)(2) in Example 1, but change the chloromethyl 2-pyridine hydrochloride in (2) to chloromethyl 4-pyridine hydrochloride.
[0033] NMR data of the resulting product:
[0034] 1 H-NMR (300MHz in CDCl 3 ): δ4.20 (6H, s, 1), 4.93 (6H, s, 6), 6.37 (6H, d, 7), 7.02 (3H, d, 2), 7.28 (6H, m, 3, 4) , 7.62(3H,d,5), 8.25(6H,d,8).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com