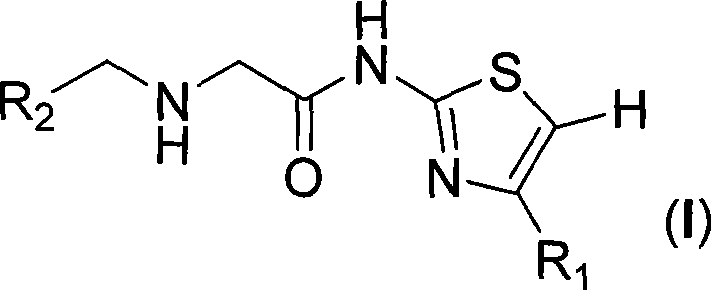
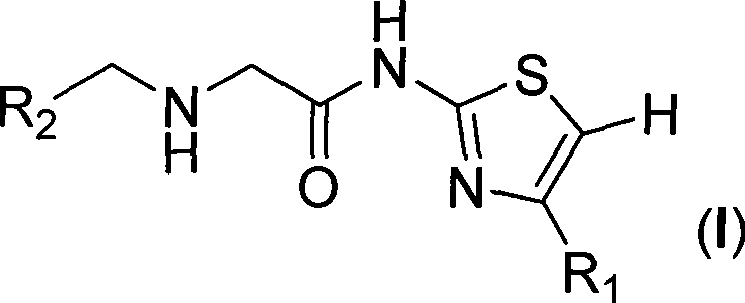
Amide thiazole derivant, preparation method and use thereof
一种乙酰基、烷基的技术,应用在糖尿病相关的药物领域,能够解决未发现体重增加等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
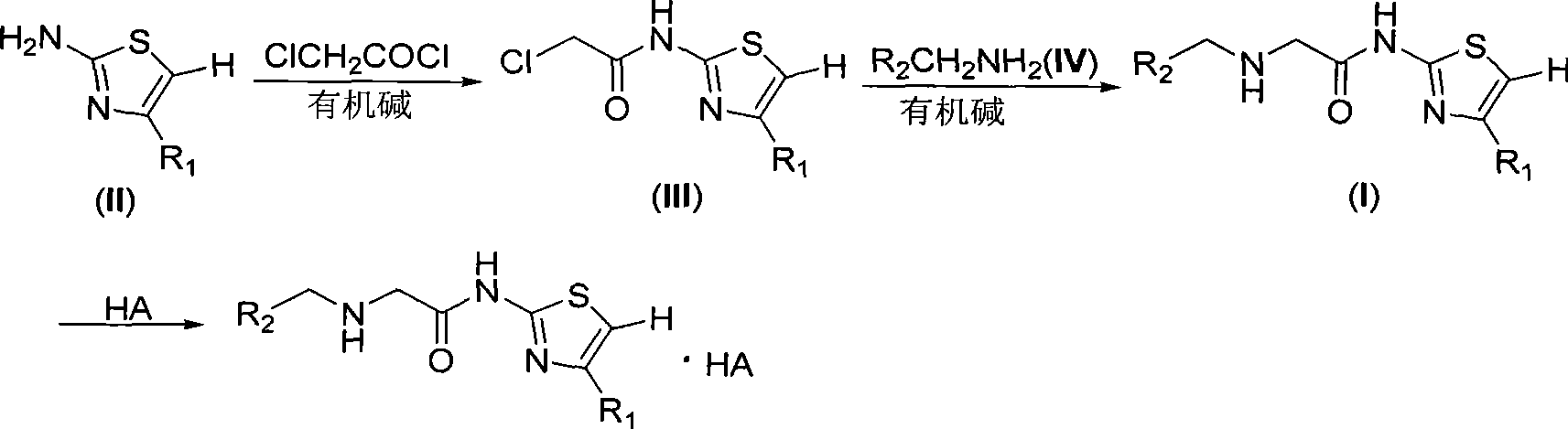
[0036] 2-(2-Chloroacetyl)amino-4-methylthiazole (III-1)
[0037]
[0038] In a 100mL round bottom flask, add 1.14g (10mmol) compound II-1, 1.31g (13mmol) dry triethylamine and 50mL dry CH 2 Cl 2 , the mixture was cooled with an ice-water bath under stirring, and then slowly added dropwise 1.24 g (11 mmol) of chloroacetyl chloride dissolved in 5 mL of dry CH 2 Cl 2 After the resulting solution was added dropwise, the reaction mixture was stirred at room temperature for 1 hour. The reaction mixture was washed with 50 mL CH 2 Cl 2 After dilution, it was washed with saturated brine, and the organic phase was washed with anhydrous Na 2 SO 4After drying, the solvent was removed on a rotary evaporator, and the obtained residue was purified by column chromatography to obtain the pure product of compound III-1, 1.75 g, with a yield of 92%. Colorless crystals, melting point 128-129°C, IR (KBr), 3185, 3053, 1653, 1578, 1380cm -1 .
[0039] Compounds II-1 and III-1 are one of ...
Embodiment 2-5
[0041] With the operation of Example 1, replace the compound II-1 in Example 1 with the compound II-2 to II-5 in the following table, and some examples use organic base diisopropylethylamine or 4-dimethylamine Basepyridine instead of triethylamine in Example 1, all the other operations are the same as in Example 1 to obtain compounds III-2 to III-5 listed in the table below.
[0042] Compounds II-2 to II-5 and compounds III-2 to III-5 belong to two classes of compounds having the general formula II and having the general formula III, respectively.
[0043] implement
Sequence
No
Yield
(%)
II
III
2
92
Diisopropyl
Ethylamine II-2: 2-Amino
III-2: 2-(2-Chloroacetyl)aminothiazole
3
90
4-Dimethylamino
pyridine II-3: 2-Amino
-4-Bromothiazole
III-3: 4-Bromo-2-(2-chloroacetyl)aminothiazole
4
...
Embodiment 6
[0054] 4-Bromo-2-{2-[(2-chlorobenzyl)amino]acetyl}aminothiazole (I-1)
[0055]
[0056] Add 1.77g (10mmol) compound III-2, 1.61g (15mmol) benzylamine (IV-1) and 1.01g (10mmol) triethylamine to a 100mL round bottom flask, then add 25mL dry THF to dissolve. The resulting reaction mixture was stirred overnight at room temperature. The reaction mixture was evaporated to remove the solvent on a rotary evaporator to obtain a crude product of compound I-1, which was purified by column chromatography to obtain a pure product of compound I-1. Colorless oil, 2.22g, yield 90%. IR(KBr), 3237, 3186, 3030, 1654cm -1 .
[0057] Compounds IV-1 and I-1 are one of the compounds having the general formula IV and the general formula I, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com