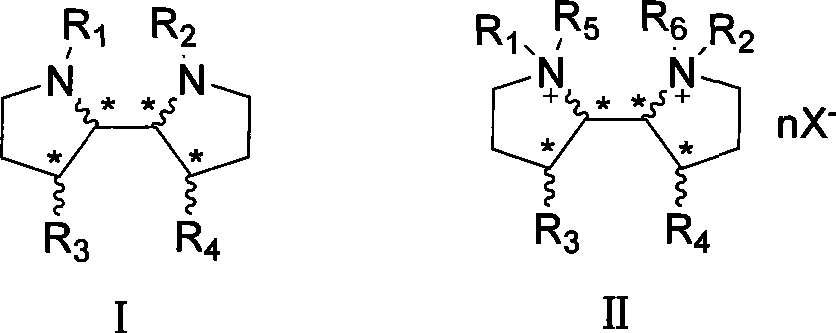
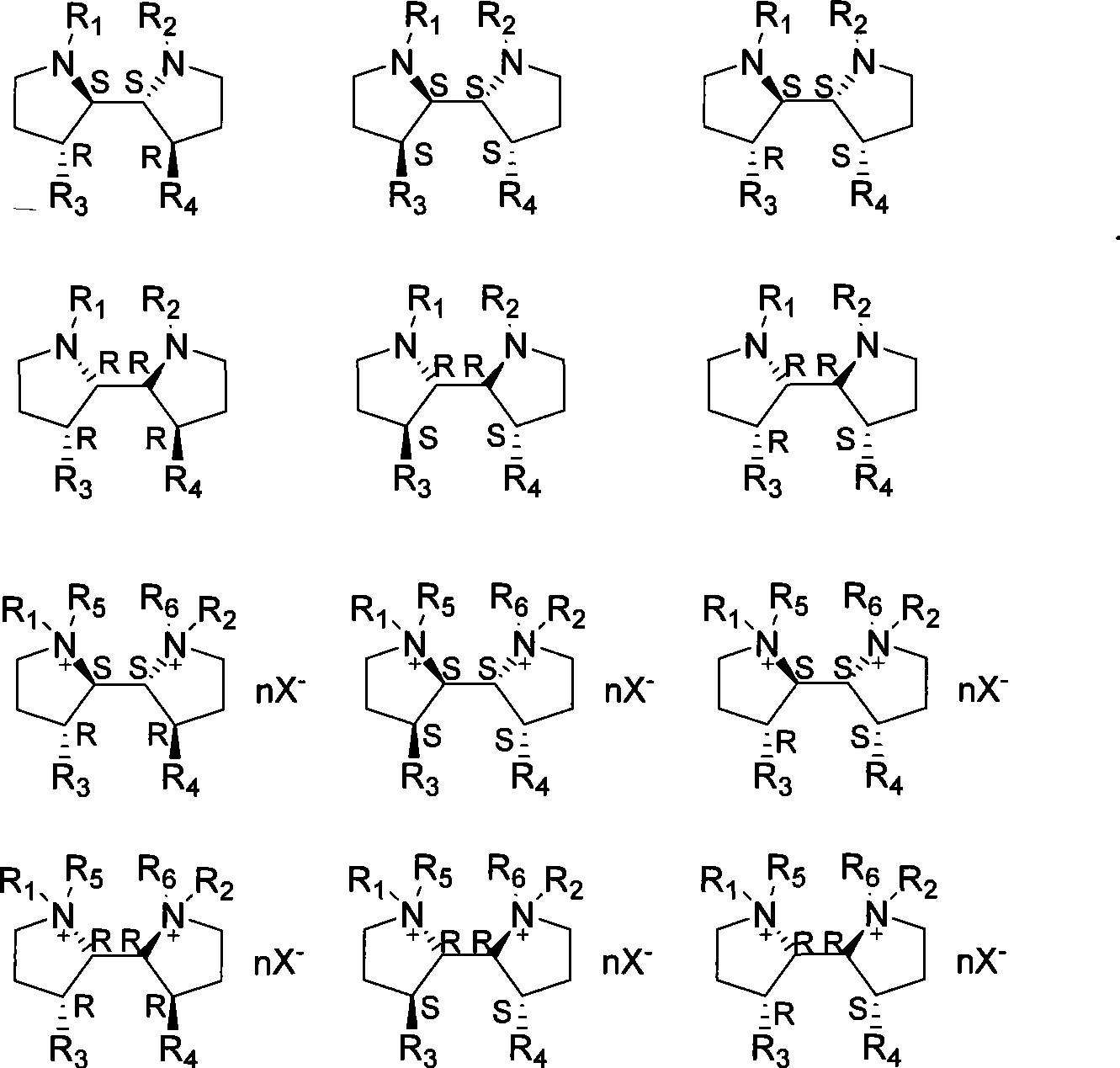
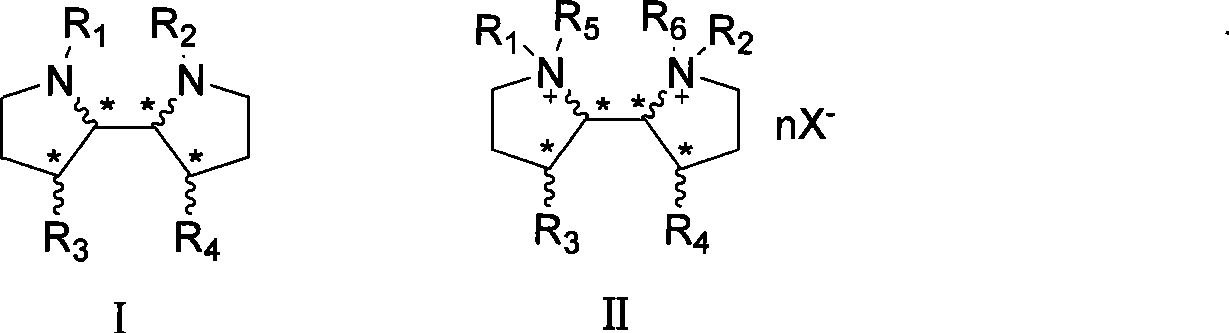
3-3'-substituted 2,2'-dipyrrolidine compounds and salts thereof
A technology of bipyrrolidine and compound, applied in the field of compound, 2, can solve the problems of unsatisfactory results of catalytic activity or stereoselectivity, and achieve the effect of high catalytic activity and stereoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] R 1 , R 2 is α-methylbenzyl, R 3 , R 4 Be benzyloxy, the stereochemistry of each chiral carbon is the same as compound Ia, and the synthetic route is as follows:
[0022]
[0023] (1) Synthetic Compound IVa from Compound IIIa
[0024] Sodium hydride (1.4g, 60%, 35.0mmol) was suspended in dry N,N-dimethylformyl (15mL), and compound IIIa (4.5g, 12mmol) was dissolved in dry N,N-dimethylformyl Formamide (10mL), slowly added dropwise to the above sodium hydride suspension in N,N-dimethylformamide under ice bath, stirred for half an hour under ice bath, then benzyl bromide (1.6mL, 35.0mmol) was slowly added under ice bath, after the dropwise addition was completed, the resulting solution was stirred at room temperature for 4 hours, after the reaction was completed, water was added under ice bath, extracted with ether, the ether layer was dried with anhydrous sodium sulfate, filtered , after concentration, the obtained crude product was purified by column chromatograph...
Embodiment 2
[0029] R 1 , R 2 for Boc, R 3 , R 4 is OH, and the stereochemistry of each chiral carbon is the same as that of compound Ia:
[0030] Compound VIa (2.7g, 4.8mmol), palladium hydroxide / carbon (1.0g), (Boc) 2 O (3.1g, 14.4mmol) was dissolved in methanol and reacted for 24 hours under a hydrogen atmosphere (20atm). The reaction solution was filtered, concentrated, and purified by column chromatography to obtain the target compound (1.8g, 96%). 1 H NMR (400MHz, CDCl 3 )δ 4.19 (br, 2H), 3.42 (br, 6H), 2.30 (br, 2H), 2.18 (br, 2H), 1.87 (br, 2H), 1.42 (s, 18H).
Embodiment 3
[0032] R 1 , R 2 for Boc, R 3 , R 4 is OBn, the stereochemistry of each chiral carbon is the same as compound Ia:
[0033] Sodium hydride (60%, 48mg, 1.2mmol) was suspended in dry DMF (4mL), and the DMF (1.5mL) solution of the target compound (0.15g, 0.40mmol) in Example 3 was added under ice-cooling, and stirred for half After 1 hour, add benzyl bromide (140 μL, 1.2 mmol), stir at room temperature, add water (15 mL) after the reaction is complete, extract with ether (20 mL×3), combine organic layers, dry over anhydrous sodium sulfate, filter, concentrate and pass through the column layer The target compound (196 mg, 89%) was purified by analysis and purification, 1 H NMR (400MHz, CDCl 3 )δ7.2~37.36(m,10H), 4.31~4.48(m,4H), 3.36~3.54(m,4H), 3.64~3.76(m,4H), 1.97~2.32(m,4H), 1.44( s, 18H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com