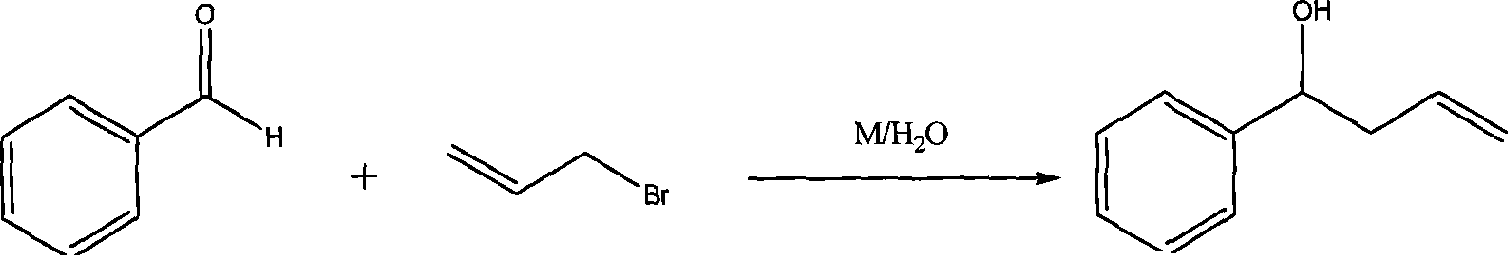Preparation method of PPh2-ordered mesopore polymer-Pd(II) heterogeneous catalyst and use thereof
A technology of heterogeneous catalyst and homogeneous catalyst, which is applied in the preparation of organic compounds, organic compound/hydride/coordination complex catalysts, chemical instruments and methods, etc., can solve the problems of environmental pollution and difficult to reuse, etc. achieve the effect of improving catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] a PPh 2 -The preparation method of ordered mesoporous polymer-Pd (II) heterogeneous catalyst, comprises the following steps:
[0027] 1) At a temperature of 308K, mix 1.10g of phenol (Phenol), 20% aqueous sodium hydroxide solution and p-PPh 2 -Phenol, 37% formaldehyde solution was stirred and mixed, and then reacted at 340K for 2h to obtain 2 Polymers with functional groups (PPh 2 -MPs) prepolymer, which is subsequently formulated into PPh with a mass ratio of 20% 2 - MPs prepolymer ethanol solution.
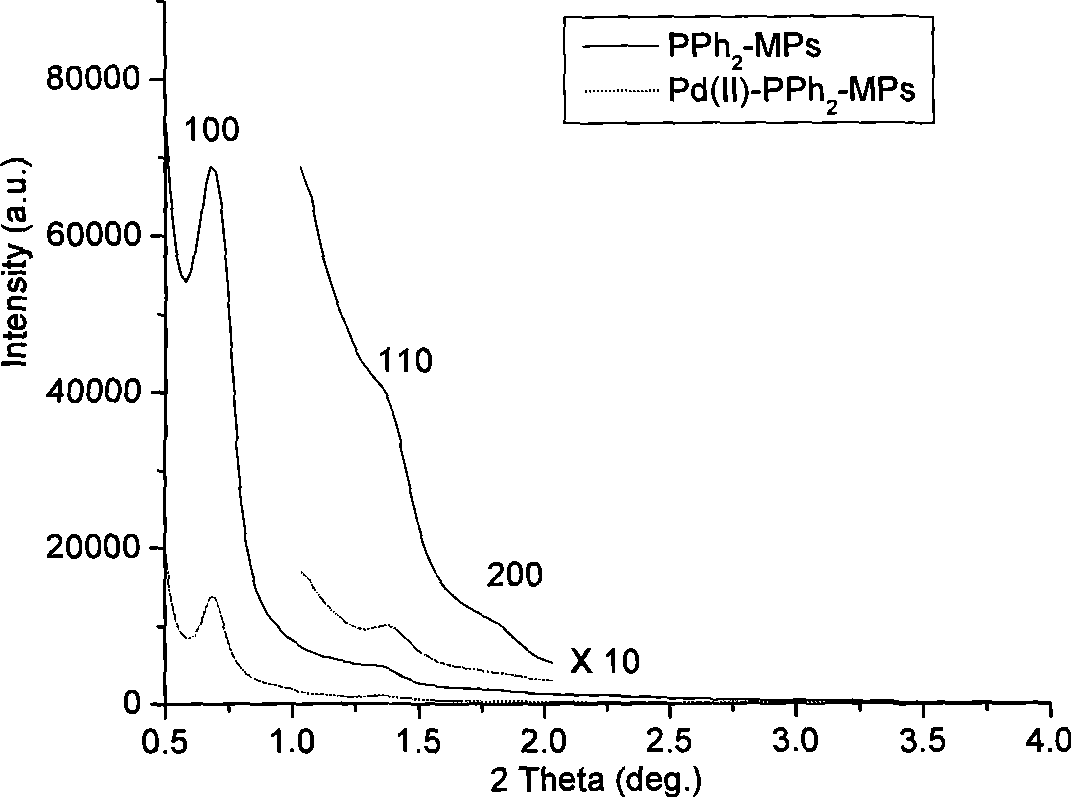
[0028] 2) PPh obtained in step 1 2 -Add 0.90g of triblock copolymer Pluronic F127 (EO106PO70EO106, M w =12,600, Acros Chemical Inc.), 20.0g ethanol solution, after standing at room temperature for 5h, further solidified at 373K temperature, 48w%H 2 SO 4 Extraction, the obtained dark brown solid is PPh 2 Ordered mesoporous polymer materials with functional groups (PPh 2 -MPs).
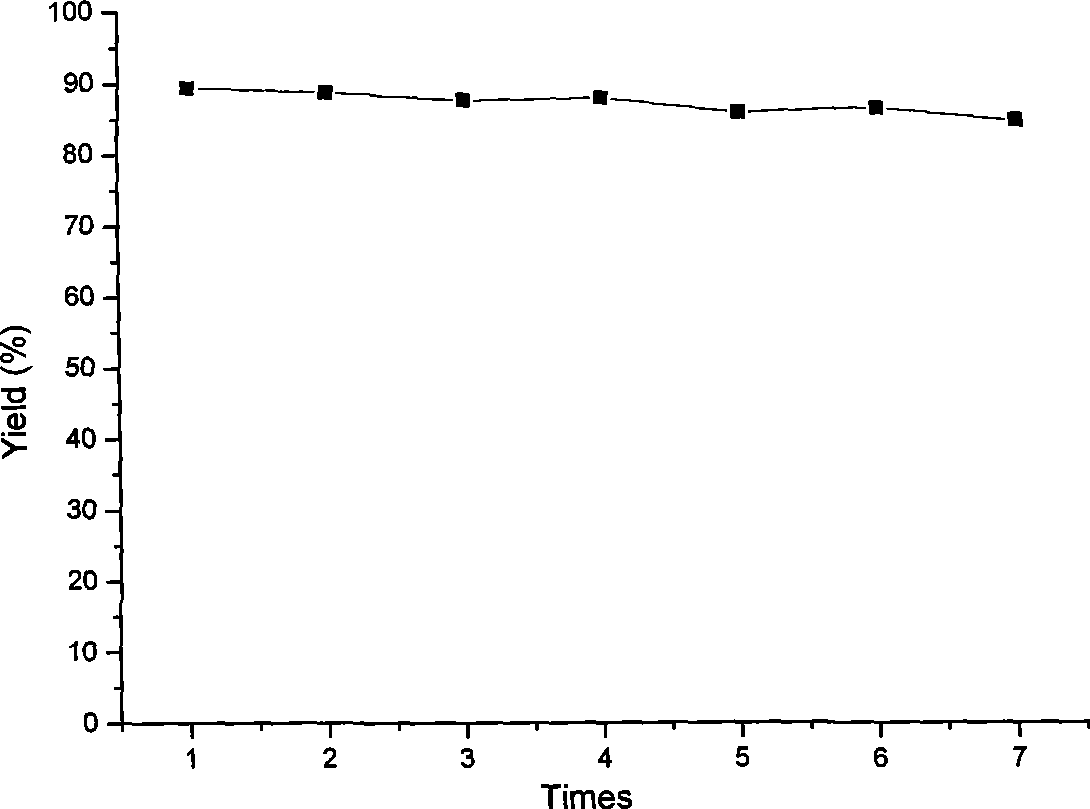
[0029] 3) Add 0.50g of PdCl 2 (PPh 3 ) 2 The homogeneous catalyst was dissolved in...
Embodiment 2
[0031] 1) At 318K, 1.19g of phenol (Phenol), 20% aqueous sodium hydroxide solution and p-PPh 2-Phenol, 37% formaldehyde solution was stirred and mixed, and then reacted at 350K for 4h to obtain 2 Polymers with functional groups (PPh 2 -MPs) prepolymer, which is subsequently formulated into PPh with a mass ratio of 20% 2 - MPs prepolymer ethanol solution.
[0032] 2) PPh obtained in step 1 2 -Add 1.10g of triblock copolymer Pluronic F127 (EO106PO70EO106, M w =12,600, Acros Chemical Inc.), 20.0g ethanol solution, after standing at room temperature for 8h, further solidified at 383K temperature, 48w%H 2 SO 4 Extraction, the obtained dark brown solid is PPh 2 Functional group ordered mesoporous polymer materials (PPh 2 -MPs).
[0033] 3) Add 0.60g of PdCl 2 (PPh 3 ) 2 The homogeneous catalyst was dissolved in anhydrous toluene, and then the PPh obtained in step 2) was slowly added 2 -MPs solid material (the molar ratio of P content in the control material to Pd content...
Embodiment 3
[0035] 1) At 310K, mix 1.15g of phenol (Phenol), 20% aqueous sodium hydroxide solution and p-PPh 2 -Phenol, 37% formaldehyde solution was stirred and mixed, and then reacted at 345K for 3h to obtain 2 Polymers with functional groups (PPh 2 -MPs) prepolymer, which is subsequently formulated into PPh with a mass ratio of 20% 2 - MPs prepolymer ethanol solution.
[0036] 2) PPh obtained in step 1 2 -Add 1.0 g of triblock copolymer Pluronic F127 (EO106PO70EO106, M w =12,600, Acros Chemical Inc.), 20.0g ethanol solution, after standing at room temperature for 7h, further solidified at 380K temperature, 48w%H 2 SO 4 Extraction, the obtained dark brown solid is PPh 2 Functional group ordered mesoporous polymer materials (PPh 2 -MPs).
[0037] 3) 0.55g of PdCl 2 (PPh 3 ) 2 The homogeneous catalyst was dissolved in anhydrous toluene, followed by the slow addition of the PPh obtained in step 2 2 -MPs solid material (the molar ratio of P content in the control material to Pd ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com