Process for synthesizing effective azolylamine derivative
A technology for pyrrole amine and derivatives is applied in the field of effective synthesis of pyrrole amine derivatives, which can solve the problems of low reaction yield, difficult purification of target products, expensive production and the like, and achieves the effect of broad application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0042] The following examples help to understand the present invention, but do not limit the content of the present invention.
[0043] Embodiment --- the general operating procedure of reaction
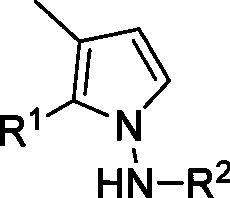
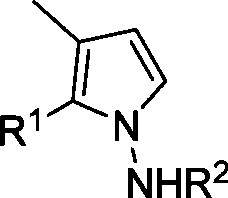
[0044] Synthesis of pyrrolamine derivatives 3 or 4: In a clean reaction tube (without any anhydrous and oxygen-free treatment), add methylenecyclopropanylaldehyde (1, 0.20mmol), hydrazide or hydrazine hydrate (2, 0.4mmol), heated to 110°C in toluene to react for ten minutes. Flash column chromatography (SiO 2 , the eluent is ethyl acetate / petroleum ether=1 / 6) to obtain the corresponding product 3 or 4.
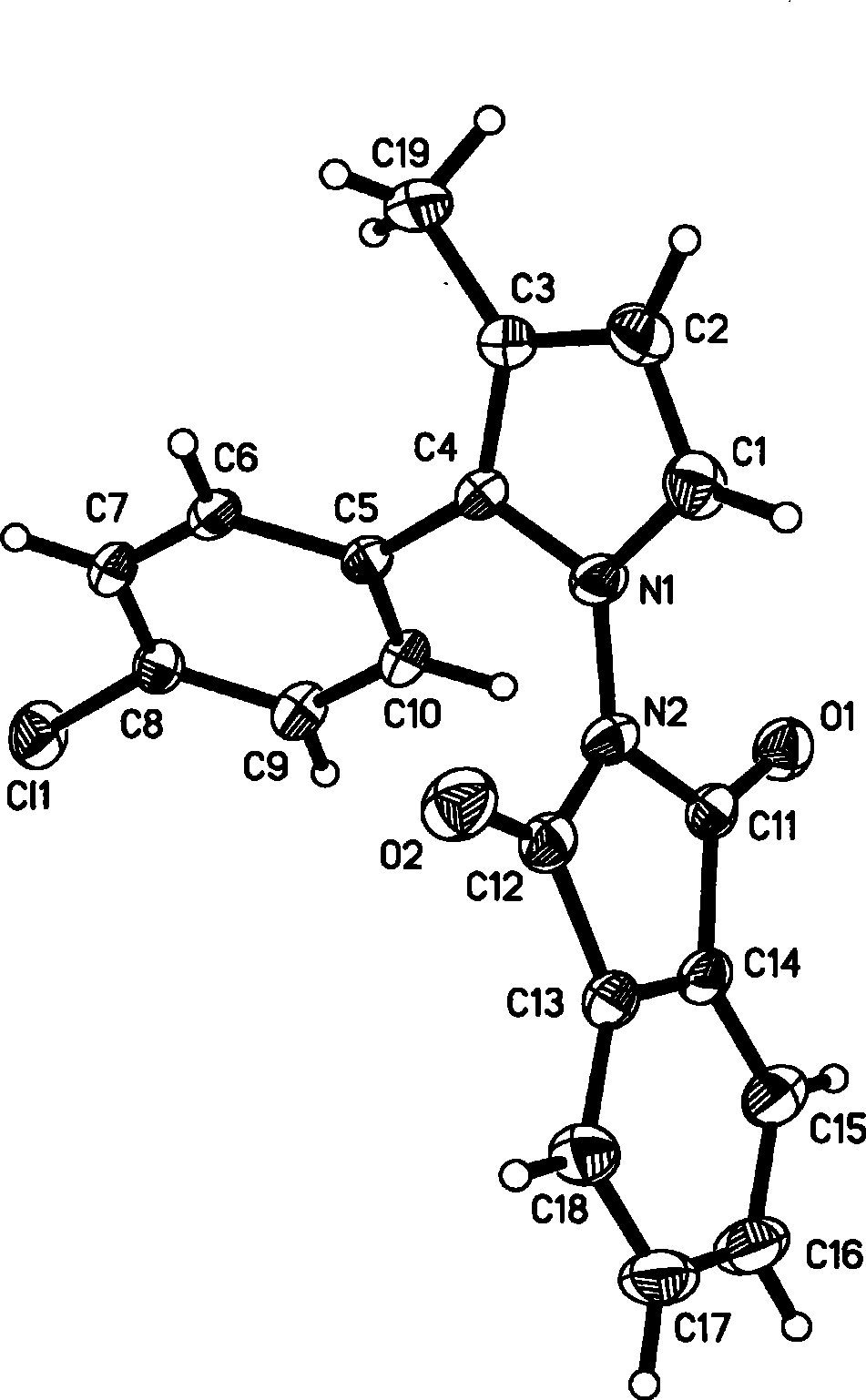
[0045] Product 3a. White solid, melting point: 193-195°C. 1 H NMR (CDCl 3 , 300MHz, TMS) δ 2.10(s, 3H, CH 3 ), 6.11(d, J=3.0Hz, 1H, CH), 6.64(d, J=3.0Hz, 1H, CH), 7.24-7.37(m, 7H, Ar), 7.46-7.51(m, 1H, Ar ), 7.60(d, J=7.5Hz, 1H, Ar), 8.76(s, 1H, NH); 13 C NMR (CDCl 3 , 75MHz, TMS).δ 12.2, 109.4, 116.0, 121.7, 127.1, 127.3, 128.2, 128.7, 129.6, 130.4, 130.9, 131.7, 132.3, 167....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com