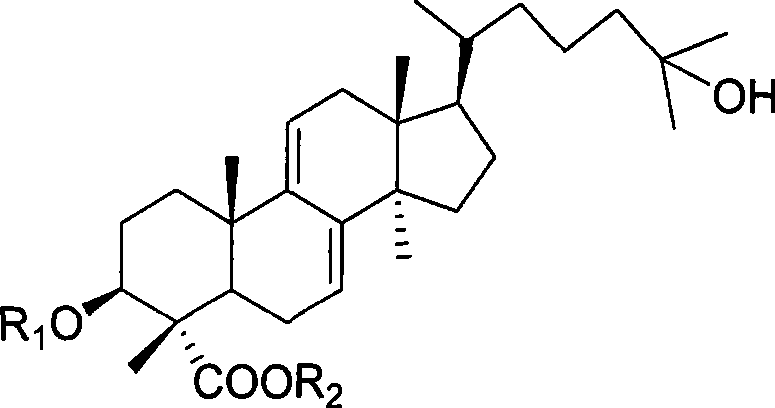
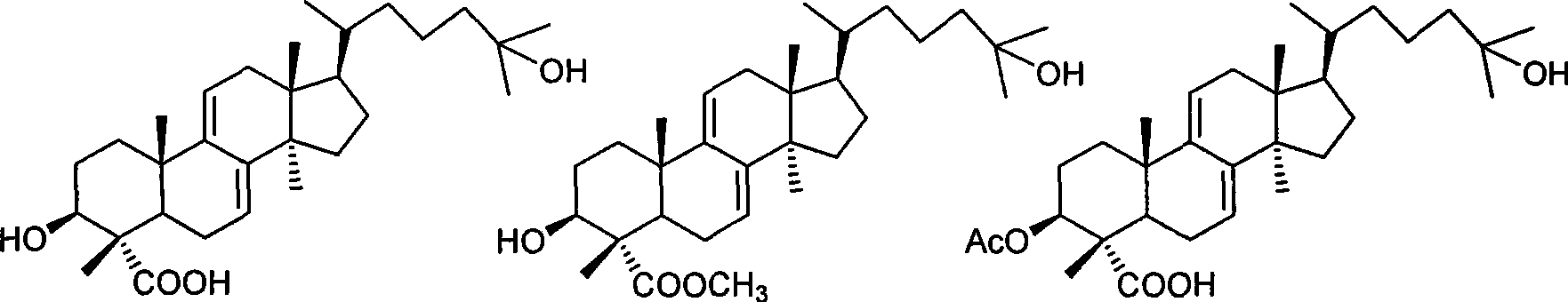
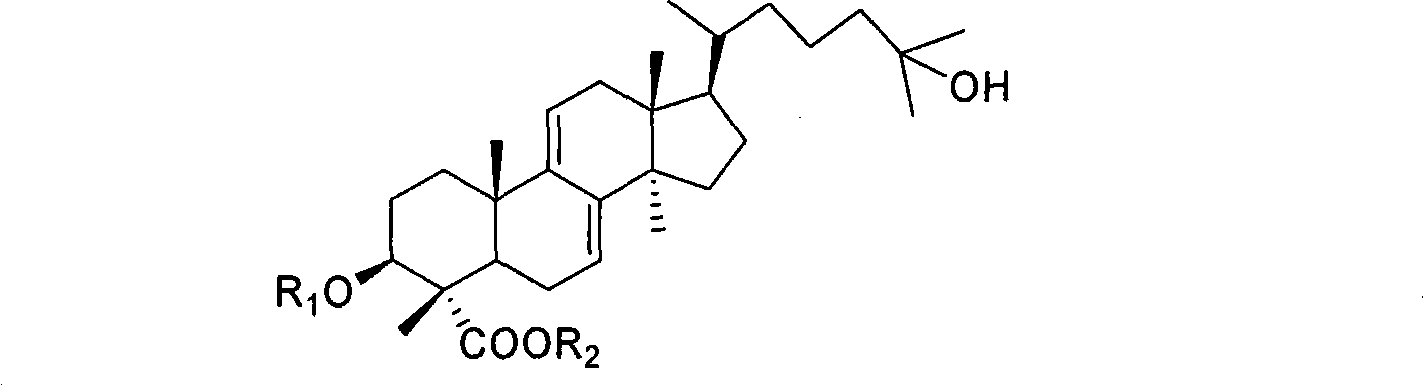
Lanoline alkane type triterpenoid sexangulic acid, derivative thereof and preparation and use thereof
A technology of triterpene compound and lanolin, which is applied in the field of medicine, can solve the problems of no galenic acid and achieve obvious anti-tumor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Preparation of lanolin-type triterpenoid lanolinic acid
[0037] (1) Extraction: The dry weight of the Chinese mangrove plant Neptonia chinensis is 3.1kg, and it is extracted 4 times by percolation with 5L of methanol. The extracts are combined and then concentrated under reduced pressure. The suspension was repeatedly extracted 3 times, and the obtained extracts were combined and concentrated under reduced pressure to obtain 41 g of ethyl acetate extract.
[0038] (2) Separation: 41g of ethyl acetate extract was subjected to 200-300 mesh silica gel column chromatography, and petroleum ether / ethyl acetate 100:0→90:10→80:20→50:50→20:80→10: 90→0:100 gradient elution, the amount of each gradient is 1000ml; among them, petroleum ether / ethyl acetate 10:90 eluted part 421mg, after 200-300 mesh silica gel column chromatography, chloroform / methanol 100:0→95: 5→90:10 gradient elution, the amount of each gradient is 200ml; 107mg of chloroform / methanol 95:5 eluted part was purifi...
Embodiment 2
[0045] Preparation of Hailianic Acid Derivatives
[0046] 1. Methyl galenic acid (R 1 for hydrogen, R 2 For the preparation of methyl)
[0047] Weigh 2.0 mg of laminic acid into a 25 mL round bottom flask, add 1 mg of CH 2 N 2 2 mL of diethyl ether solution, reacted at room temperature for 18 h, removed diethyl ether and CH under reduced pressure 2 N 2 , to get methyl heliolate (R 1 = H, R 2 =CH 3 ).
[0048] 2. 3-Acetoxypiplenic acid (R 1 for Ac, R 2 for the preparation of hydrogen)
[0049] Weigh 2.0 mg of niporinic acid in a 25 mL round-bottomed flask, add 1.5 mL each of anhydrous pyridine and acetic anhydride, react at room temperature for 18 h, remove pyridine and acetic anhydride under reduced pressure to obtain 3-acetoxypicillinic acid (R 1 =Ac,R 2 = H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com