Gene engineering monoclonal antibody combined with A-beta oligomer specificity
A monoclonal antibody and oligomer technology, applied in the direction of antibodies, drug combinations, microorganism-based methods, etc., can solve the problem that the spatial three-dimensional antigen epitope has not been clearly explored, and achieve strong activity, easy screening, and reduced toxicity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1A-be
[0049] Example 1A-Preparation of beta oligomers
[0050] A-beta monomer (purchased from American Peptide, USA) was dissolved in HFIP (hexafluoroisopropanol, purchased from Sigma) to prepare a solution with a concentration of 1 mg / ml. Ultrasonic treatment in a water bath at room temperature for 10 minutes, aliquoted into 1.5ml centrifuge tubes, placed in a fume hood to allow HFIP to completely evaporate, and stored at -20°C for later use.
[0051] After the above-treated A-beta was equilibrated at room temperature for 10 minutes, DMSO (dimethyl sulfoxide, purchased from Sigma) was added to fully dissolve A-beta, and the final concentration was 1 mg / ml. A certain amount of A-beta was added to pH 7.4 PBS buffer to make A-beta concentration 10 μM. The A-beta solution was incubated at 37° C. for 12 hours, centrifuged at 14,000 rpm for 20 minutes, and the bottom precipitate was discarded to obtain the A-beta oligomer-containing supernatant. The formation of A-beta oligomers in the...
Embodiment 2
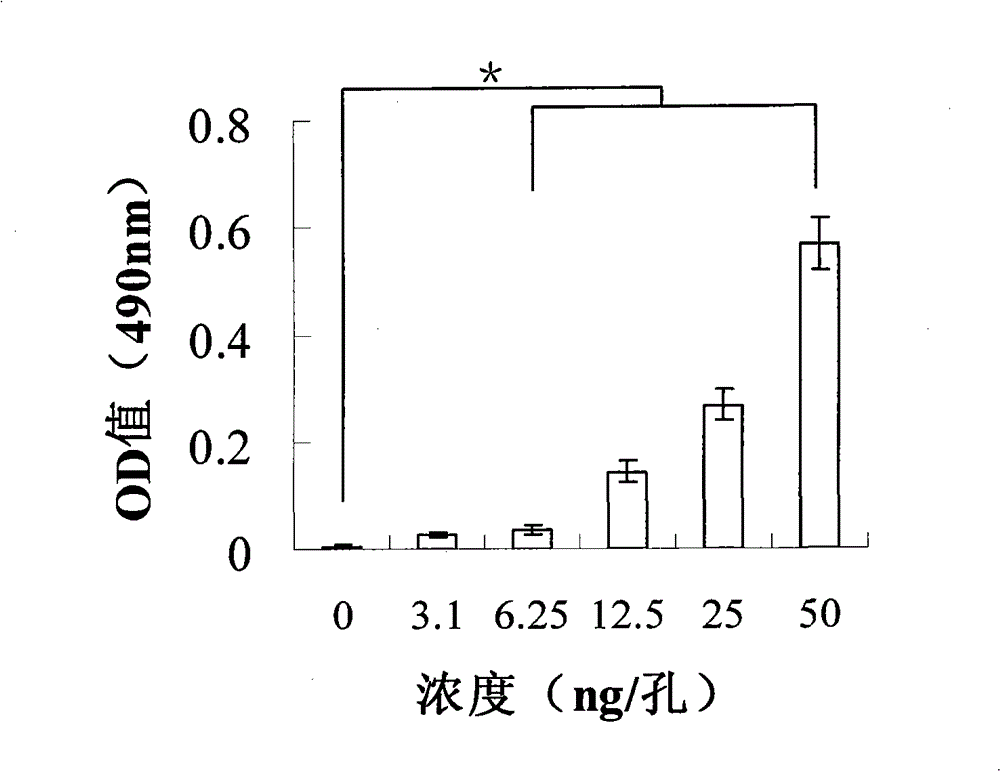
[0052] Example 2 Screening of positive clones
[0053] The A-beta oligomer obtained in Example 1 was diluted to 10-100 μg / mL with coating buffer (PBS, pH=7.4), 4 mL was added to the immunotube, and coated overnight at 4°C. The supernatant was discarded and the tube was quickly washed 3 times with PBS. The immunotubes were filled with 3% BSA and blocked vertically for 2 h at room temperature. The supernatant was discarded and the tube was quickly washed 3 times with PBS. The phage antibody library (purchased from MRC Centre, UK) was suspended in 4 mL of 3% BSA and added to the immunotube, incubated upside down for 1 h at room temperature, and then incubated vertically for 1 h. 10 washes with 0.1% Tween-20 in PBS (20 washes for the second round of screening and beyond). After the PBS was blotted dry, 500 μL of trypsin-PBS solution was added to elute the phage, and the cells were incubated upside down for 10 min at room temperature. 250 μL of the eluted phage was added to 1.7...
Embodiment 3
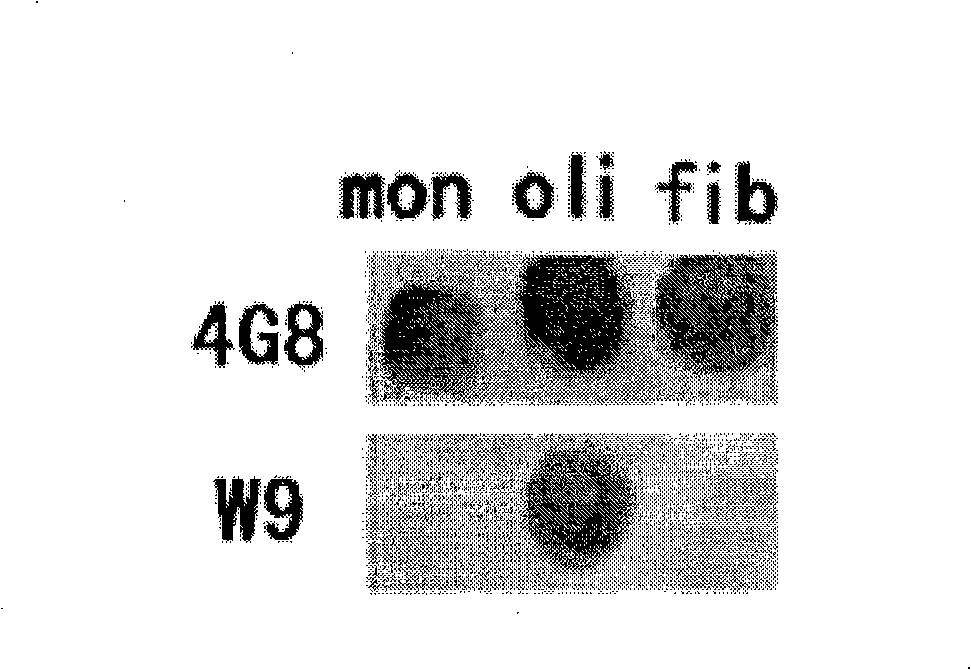
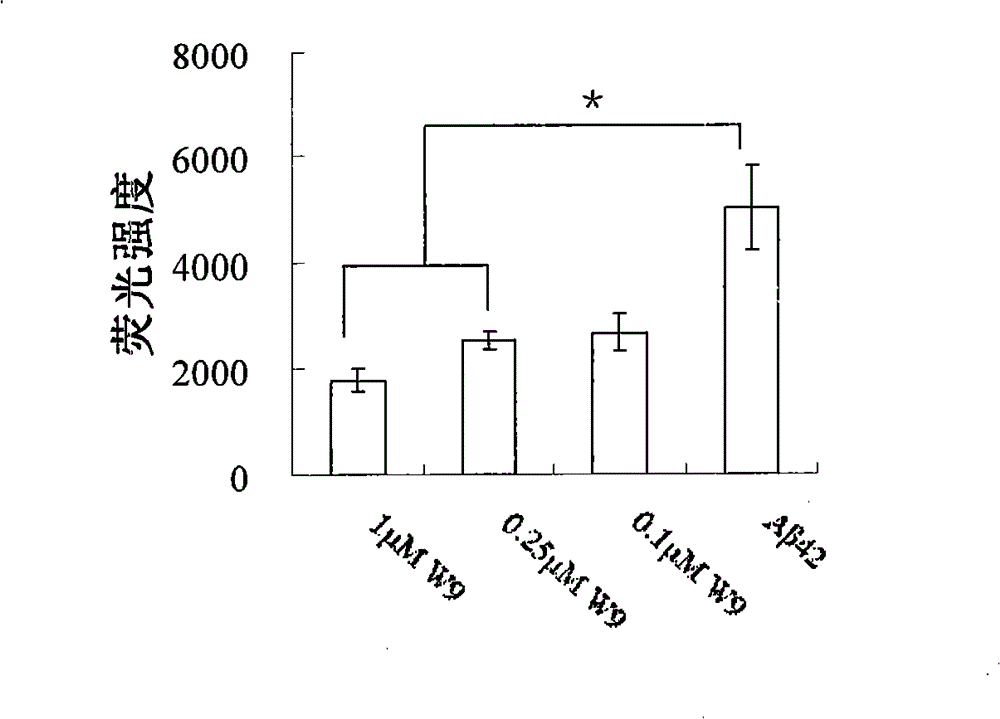
[0060] Example 3 Identification of Antibodies
[0061] Abeta monomers, oligomers and fibrils (Abeta monomers were incubated at 37°C for more than 4 days and verified by atomic force microscopy) were spotted onto 3 μL of NC (nitrocellulose) membranes respectively. After blocking the membrane with 5% BSA, scFv W9 was added to incubate for 1 hour, and the membrane was washed three times with PBS for 5 minutes each. Then, 1:5000 diluted Protein A (purchased from Santa Cruz, USA) was added to incubate for 1 hour, and the membrane was washed three times with PBST (Tween-20 concentration of 0.1%) for 5 minutes each time, and color was developed with DAB. Only clones showing spots at A-beta oligomers, not A-beta monomers and fibrils were the desired clones.
[0062] The above positive clones are sequenced and identified, and the clones that conform to the basic structure of the antibody in the antibody library are complete single-chain genetically engineered antibodies. The sequenci...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com