Process for producing high-purity prasugrel and acid addition salt thereof
A technology of prasugrel hydrochloride and acid addition salt, which is applied in the directions of pharmaceutical formulations, organic active ingredients, medical preparations containing active ingredients, etc., can solve the problem of not describing the method of reducing by-product OXTP and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0115] The embodiment that uses free prasugrel to prepare high-purity prasugrel hydrochloride
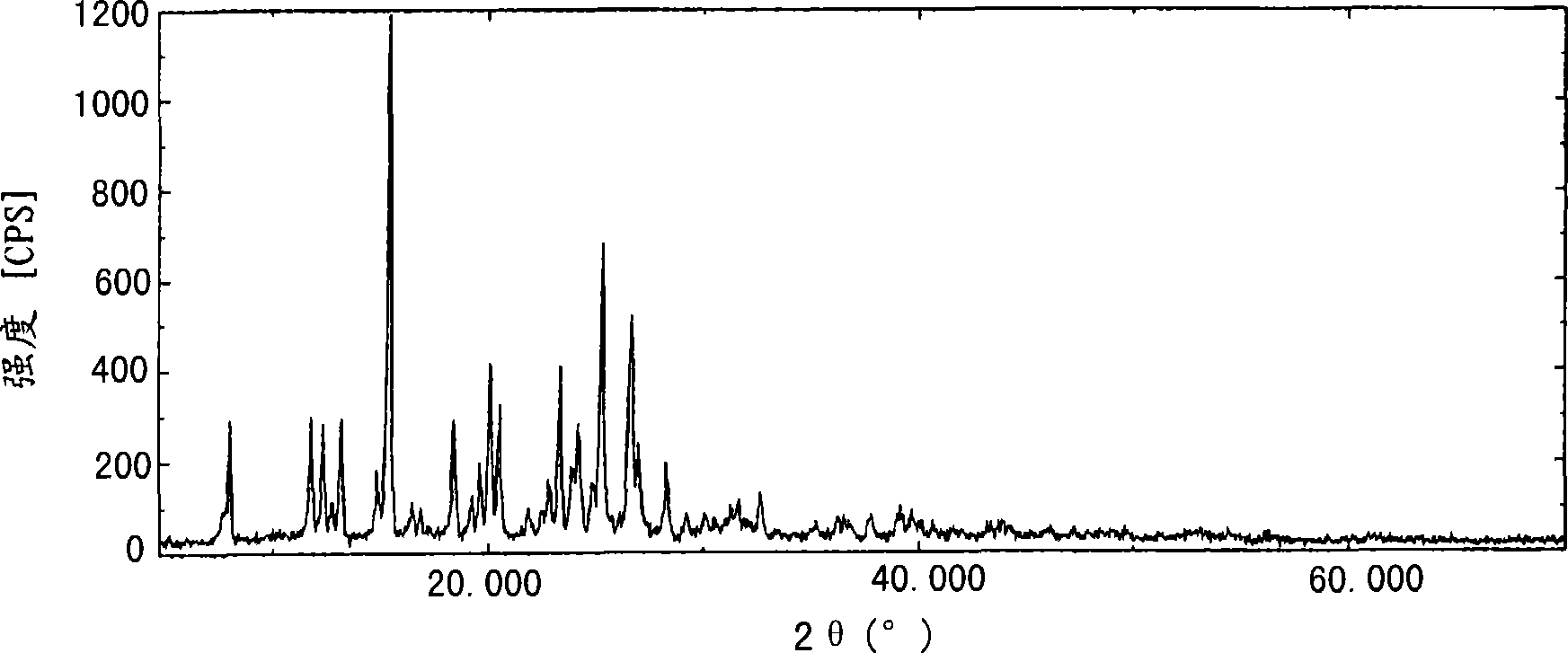
[0116] To 8.00g of 2-acetoxy-5-(α-cyclopropylcarbonyl-2-fluorobenzyl)-4,5,6,7-tetrahydrothieno[3,2-c]pyridine and 398 mg of activated clay was added to 43 g of acetone, and the resulting mixture was stirred at 32°C. The reaction solution was filtered, the residue was washed with 4.41 g of acetone, and then 1.12 g of 36% concentrated hydrochloric acid was added dropwise to the solution at 52°C over a period of 1 minute. 238 mg of Crystal B2 obtained by the method described in JP2002-145883 was added thereto as a seed crystal, and the obtained mixture was then stirred at the same temperature for one hour. Further, 1.07 g of 36% concentrated hydrochloric acid was added dropwise thereto over a period of one hour, and then the obtained mixture was stirred at 40°C for 2 hours and further stirred at 30°C for 1 hour. Precipitated crystals were collected by filtration, washed with 15.8 g o...
Embodiment 2
[0126] The embodiment that uses free prasugrel to prepare high-purity free prasugrel
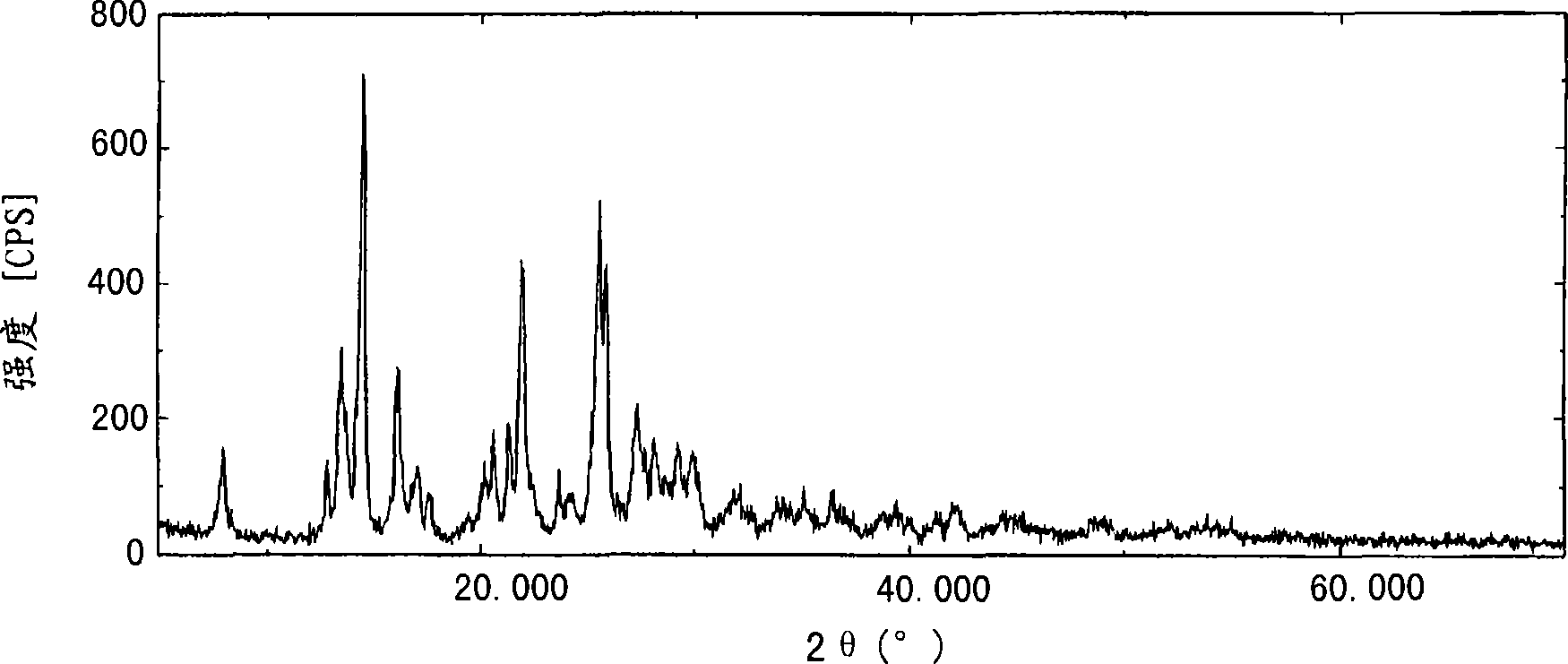
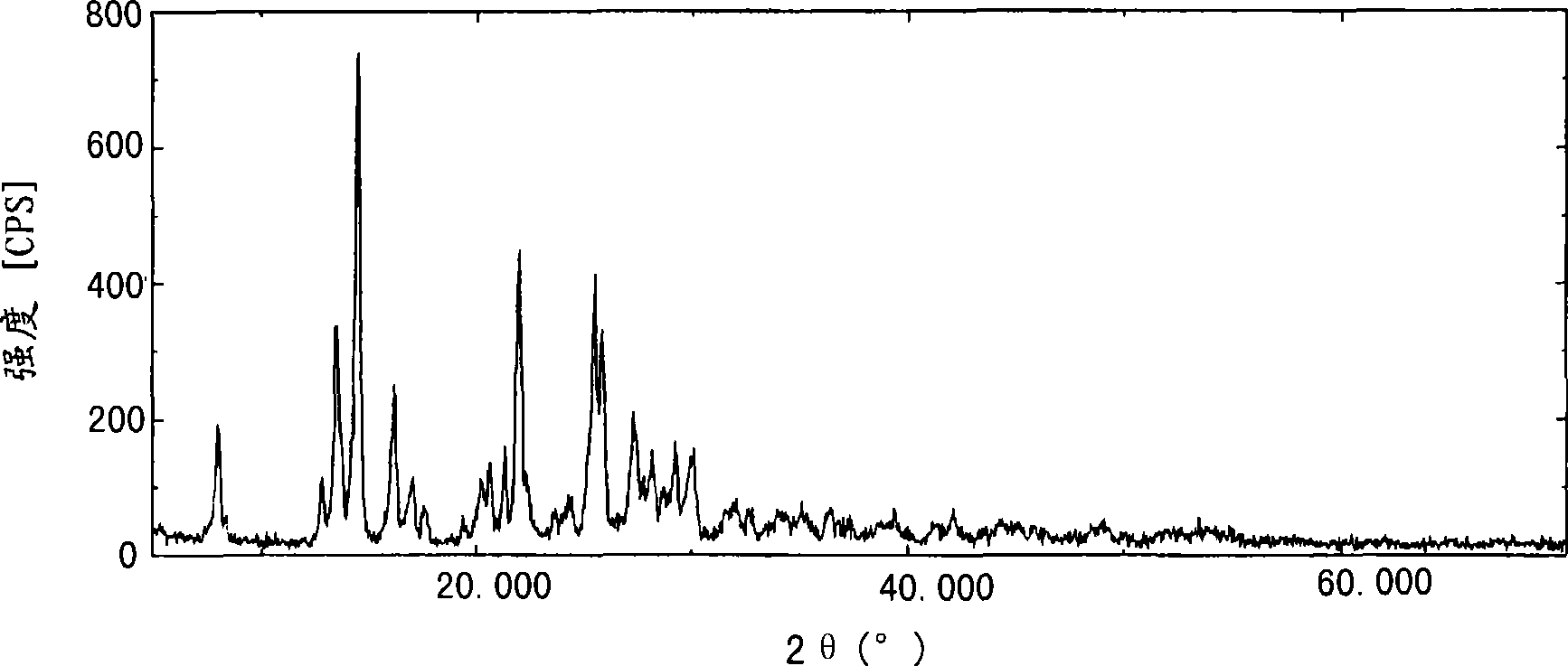
[0127] To 7.00 g of Compound (I) was added 46.3 g of acetonitrile, and the resulting mixture was stirred at 40°C for 10 minutes, and then the reaction solution was cooled to -15°C. Thereto was added dropwise 29.4 g of water precooled to the same temperature over a period of 35 minutes, and then the resulting mixture was stirred at the same temperature for 30 minutes. Precipitated crystals were collected by filtration, washed with 10.5 g of a precooled acetonitrile-water mixed solvent, and dried at 45° C. under reduced pressure for 5 hours to provide 6.50 g of the title compound.
[0128] The liquid chromatography of the obtained high-purity free prasugrel is as follows: Figure 6 shown.
[0129] Figure 6 The measurement conditions in are as follows.
[0130] (Measurement Conditions) Detector: Ultraviolet Absorption Meter (Measurement Wavelength: 240nm)
[0131] Analytical column: Caden...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com