Nicorandil freeze-dried injection and preparation method thereof
A technology of freeze-dried powder injection and nicorandil, applied in the field of nicorandil freeze-dried powder injection and its preparation, can solve acute clinical consequences and other problems, and achieve the effect of high efficiency, energy saving and drug stability in the preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Composition (1000 made in total):
[0017] Nicorandil 12g
[0018] Mannitol 18g
[0019] Sodium citrate solution
[0020] Add water for injection to 1500ml
[0021] Preparation Process
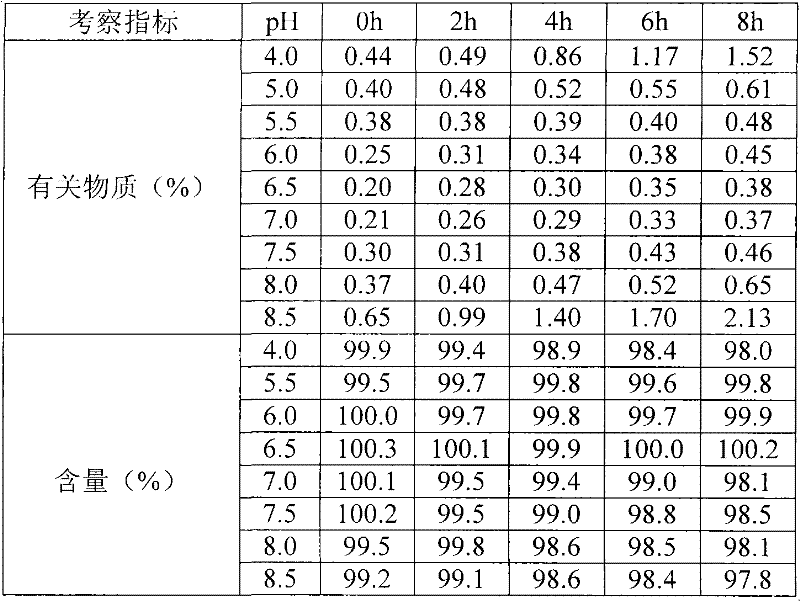
[0022] 1. Preparation: Take an appropriate amount of water for injection and add mannitol to dissolve it completely, add activated carbon, absorb for 30 minutes, and filter to remove carbon. Add the prescribed amount of Nicorandil below room temperature to dissolve, adjust the pH to 5.5-7.5 with sodium citrate solution, add water for injection to the full amount to obtain an intermediate solution, test, and sterilize and filter.
[0023] 2. Filling: Determine the filling volume according to the determination result of the intermediate content, carry out filling and half stoppering;
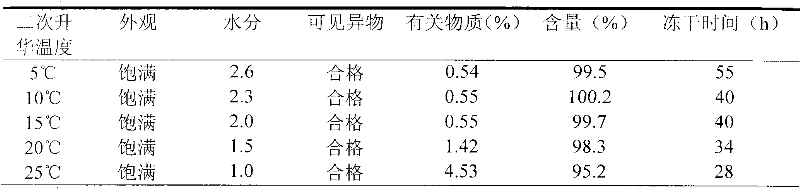
[0024] 3. Freeze-drying: freeze-drying parameters and conditions
[0025] 3.1 Turn on the cooling of the front box to reduce the temperature of the heat transfer oil to below -25°C, keep it for a peri...
Embodiment 2
[0032] Composition (1000 made in total):
[0033] Nicorandil 48g
[0034] Dextran 72g
[0035] Sodium citrate solution
[0036] Add water for injection to 6000ml
[0037] The preparation process is the same as in Example 1, wherein the pH value of the intermediate solution is adjusted to 6.3, and the secondary sublimation temperature is 10°C.
[0038] After testing the intermediate solution and finished product obtained by the above method, the related substances were 0.31% and 0.53% respectively.
Embodiment 3
[0040] Composition (1000 made in total):
[0041] Nicorandil 48g
[0042] Mannitol 50g
[0043] Dextran 22g
[0044] Sodium bicarbonate-sodium carbonate solution Appropriate amount
[0045] Add water for injection to 5000ml
[0046] The preparation process is the same as in Example 1, wherein the pH value of the intermediate solution is adjusted to 6.5, and the secondary sublimation temperature is 10°C.
[0047] After testing the intermediate solution and finished product obtained by the above method, the related substances were 0.28% and 0.54% respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com