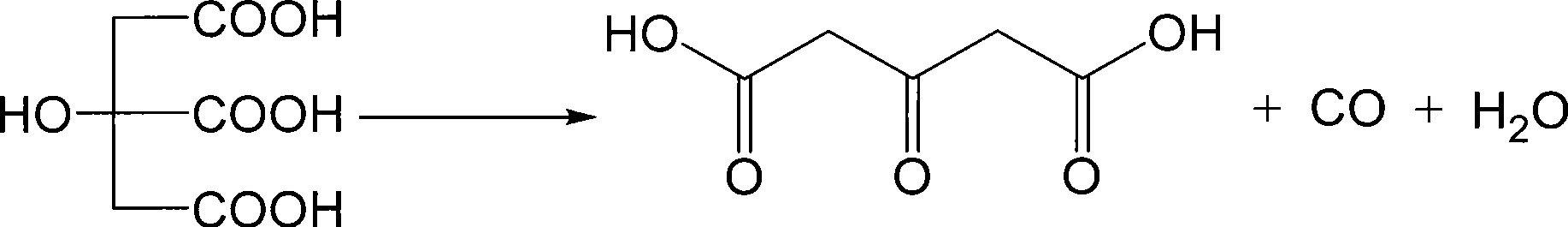
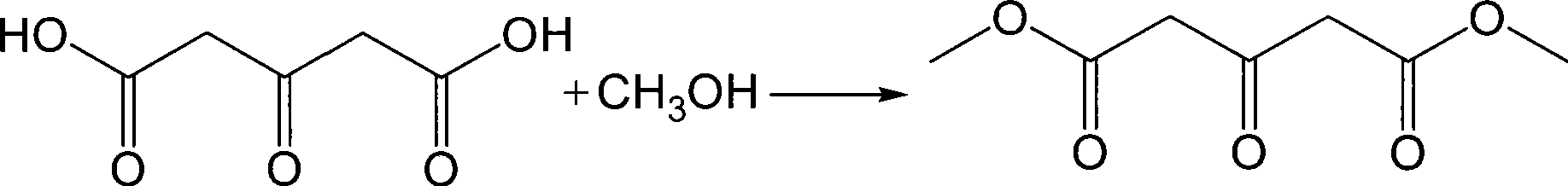
Preparation of dimethyl acetone-1,3-dicarboxylate
A technology of dimethyl acetone dicarboxylate and acetone dicarboxylic acid, applied in the field of preparation of dimethyl acetone dicarboxylate, can solve the problems of unfavorable industrialized production, poor production safety, high equipment requirements, and achieve outstanding substantive characteristics , to ensure production safety, high purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Add 400mL of concentrated sulfuric acid into a 1000mL three-neck flask, heat to 65°C, add 384g (2.0mol) of citric acid in batches, keep the reaction at 50°C for 1hr after the addition, and cool down to below 20°C. Add dropwise into 500mL of water, cool to keep the temperature of the system not higher than 35°C, stir and cool to below 10°C after dropping, filter the precipitated crystals, rinse the filter cake with cold water, and dry to obtain 203.7g of acetonedicarboxylic acid with a yield of 69.8% , content 98%.
Embodiment 2
[0028] Add 146g (1.0mol) of acetone dicarboxylic acid prepared in Example 1 and 600mL of anhydrous methanol into a 1000mL three-necked flask, stir and cool to below 20°C, add 130.9g of thionyl chloride dropwise, and reflux for 2 hours after the addition is complete. , then distill off most of the methanol (about 420mL), cool to below 20°C, add dropwise to 500mL of cold water, and control the temperature below 20°C. Stand and separate layers, extract the organic phase with 300mL dichloromethane, combine the dichloromethane layers, wash with water, saturated sodium bicarbonate solution, and water until neutral, dry with anhydrous sodium sulfate, and recover dichloromethane by atmospheric distillation , and then collect the 105°C / 500Pa fraction under reduced pressure to obtain 158g of a white powder product, with a yield of 90.8%, a content of 98.6%, an acidity≤0.3%, and a melting point of 126-127°C.
Embodiment 3
[0030] Add 400L of concentrated sulfuric acid into a 1000L reactor, heat to 65°C, add 384kg (2.0kmol) of citric acid in batches, keep the reaction at 50°C for 1.5hr after the addition, and cool down to below 20°C. Add dropwise into 500L of cold water, cool to make the system temperature not higher than 35°C, stir and cool to below 10°C after dropping, centrifuge the precipitated crystals, rinse with cold water, and dry to obtain 201.5 kg of acetonedicarboxylic acid, with a yield of 69%. Content 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com