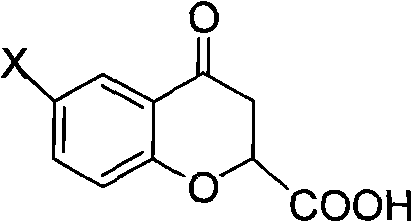
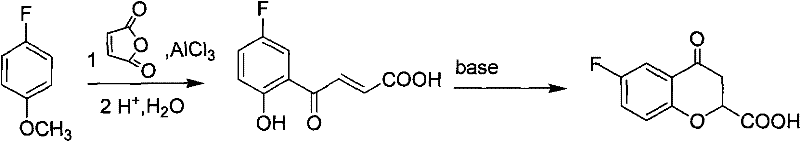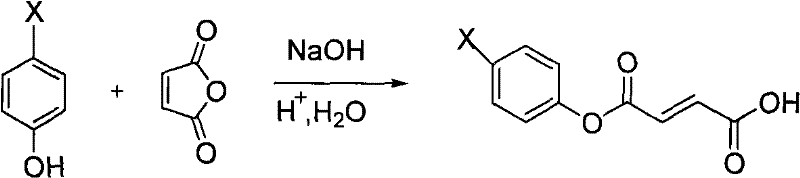Preparation of 6-substituted-4-chromanone-2-carboxylic acid
A technology of chromanone and formic acid, applied in the direction of organic chemistry, can solve the problems of consumption, unfavorable industrialization, high price, etc., and achieve the effect of optimizing the process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1, a kind of preparation method of 4-chromanone-2-formic acid, carries out following steps successively:
[0039] 1), phenolic esterification:
[0040] Add 4.0g of phenol and 50ml of 1mol / L sodium hydroxide solution into a round-bottomed flask, stir and add 2.40g of maleic anhydride at room temperature for reaction, TLC traces until the reaction of the raw materials is complete, and the reaction time is 1h.
[0041] Pour the resulting reaction mixture into a mixed system of hydrochloric acid and ice (composed of 1mol / L100ml hydrochloric acid and ice), filter, wash the resulting filtrate with water, and dry it under an infrared lamp to obtain a white solid, that is, 3-phenyl- 3-oxo-2-butenoic acid 4.4 g.
[0042] 2), carboxyl esterification:
[0043] Put the obtained product of step 1) and p-toluenesulfonic acid 3g, 12ml benzene, and 4ml ethanol into a round-bottomed flask, and install an oil-water separation device on the round-bottomed flask; The organic ...
Embodiment 2
[0049] Embodiment 2, a kind of preparation method of 4-chromanone-2-formic acid,
[0050] Change 2.40g maleic anhydride into 3.53g in the step 1) of embodiment 1, change 1mol / L sodium hydroxide 50ml into 75ml, and other reaction conditions are all equal to embodiment 1 (therefore, the mole between all the other raw materials Than relation is with embodiment 1).
[0051] 2.77 g of the product 4-chromanone-2-carboxylic acid was obtained with a purity of 100% and a yield of 40.1%.
Embodiment 3
[0052] Embodiment 3, a kind of preparation method of 4-chromanone-2-formic acid,
[0053] The 2.40g maleic anhydride in the embodiment 1 step 1) is changed into 4.00g, and 1mol / L sodium hydroxide 50ml is changed into 90ml, and other reaction conditions are all equal to embodiment 1.
[0054] 2.73 g of the product 4-chromanone-2-carboxylic acid was obtained with a purity of 98.0% and a yield of 34.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com