Production method of O, O-dialkyl thiophosphoryl chloride
A technology of alkyl phosphorothioate and dimethyl phosphorothioate, applied in the production field of O, O-dialkyl phosphorothioate chloride, which can solve the problems of many by-products, large environmental pollution, and only , to achieve the effect of large cooling consumption and large heat release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
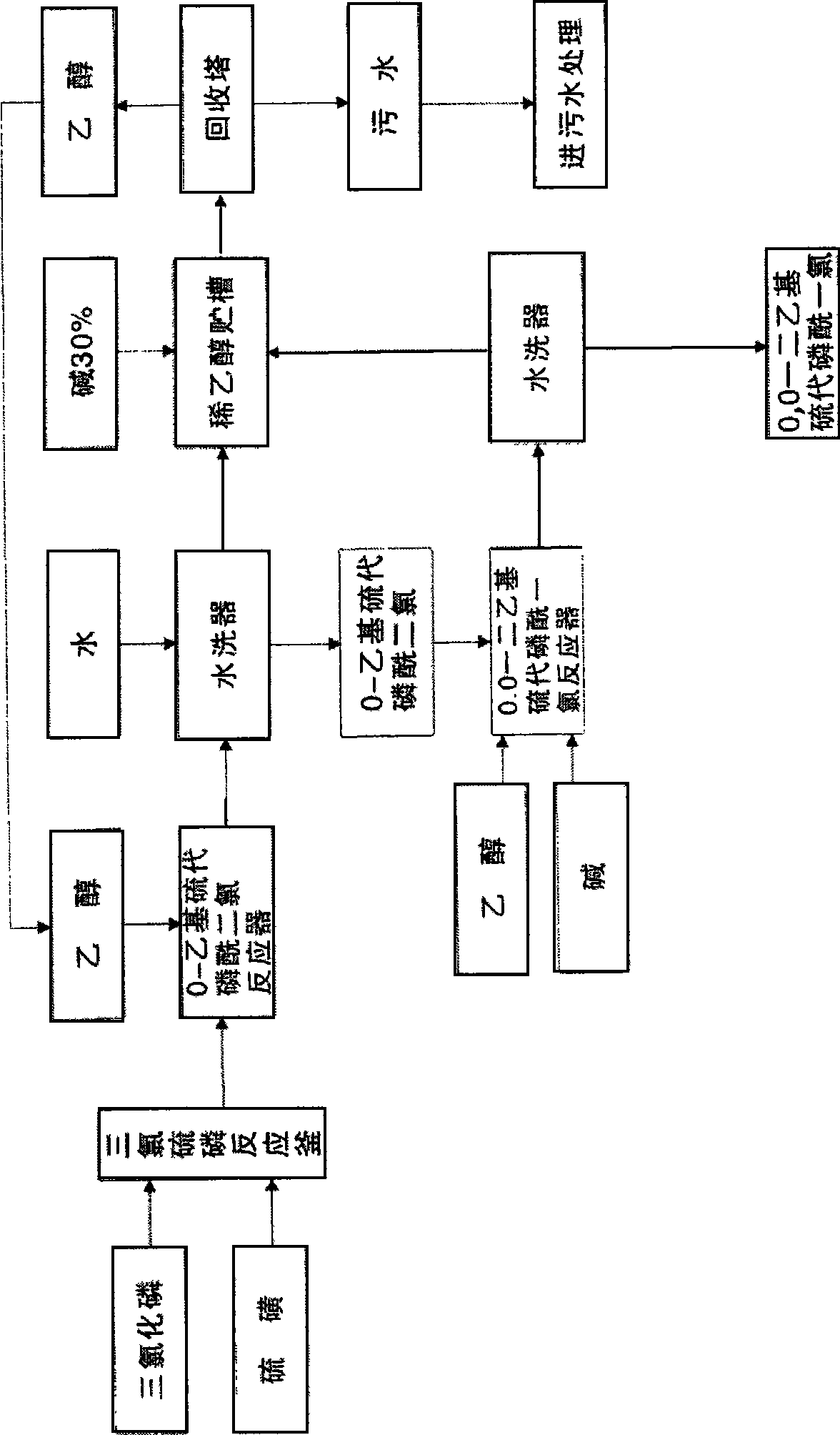
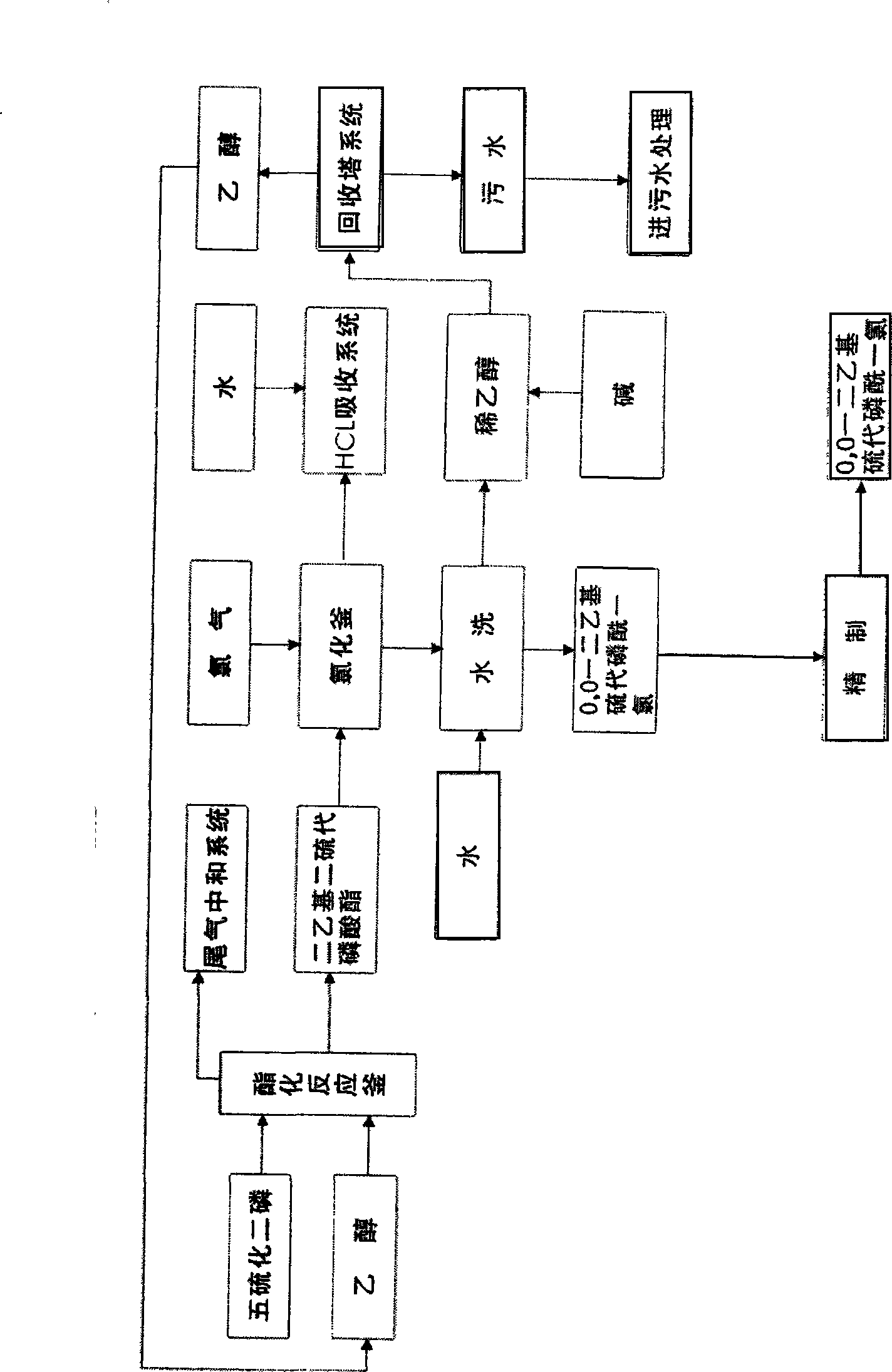
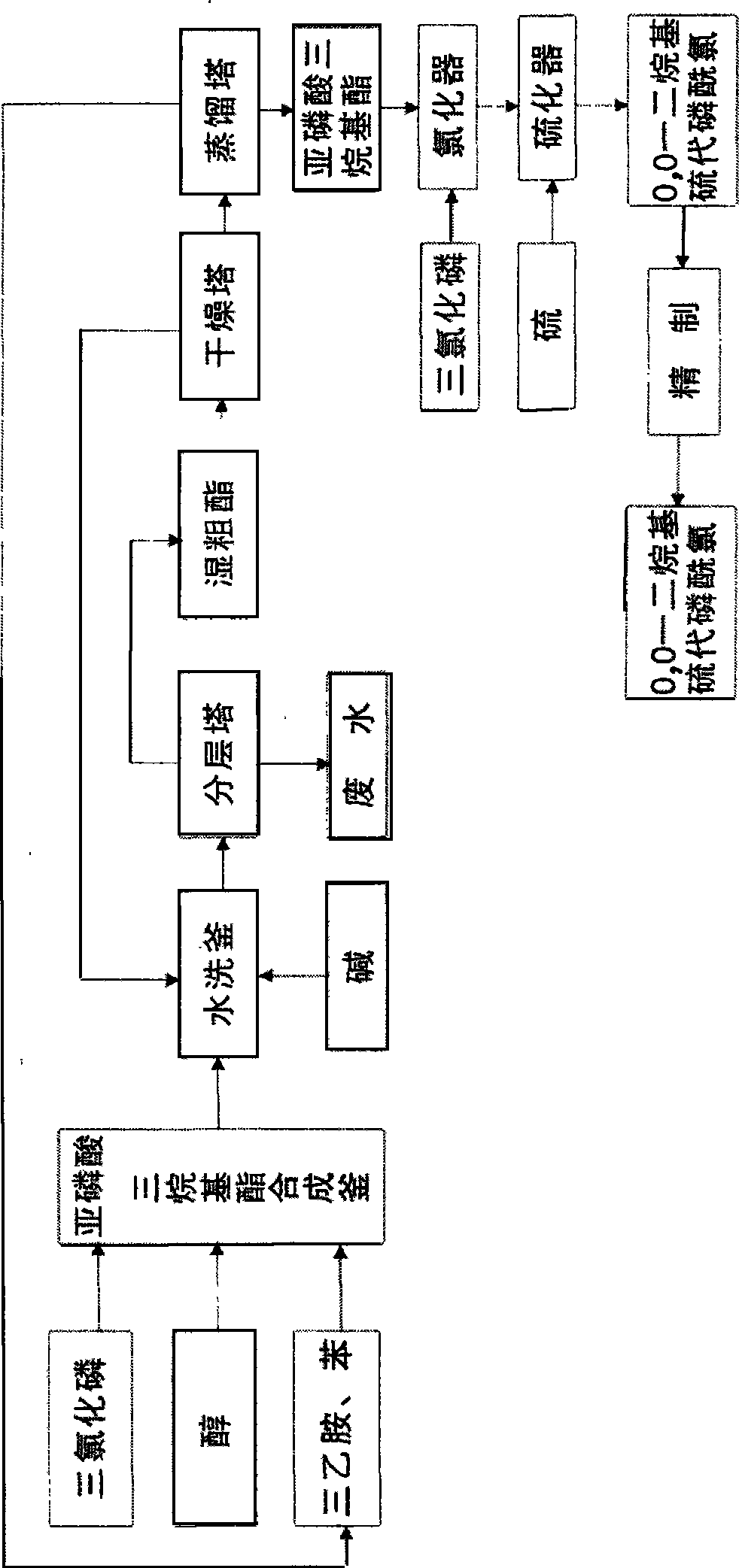
[0055] Embodiment 1: The raw materials used in the synthesis of this product are phosphorus trichloride, anhydrous methanol, sulfur, first use phosphorus trichloride and anhydrous methanol in the presence of solvent benzene and acid-binding agent triethylamine in trialkyl synthesis The reaction in the kettle generates trimethyl phosphite, and the trimethyl phosphite reacts with phosphorus trichloride in the chlorinator to generate O, O-dimethyl phosphorus monochloride, and then O, O-dimethyl-phosphorous monochloride Phosphorus chloride and sulfur react in the sulfide to produce crude O, O-dimethylphosphoryl thiochloride, and after post-treatment, the fine product of O, O-dimethylphosphoryl thiochloride can be obtained.
[0056] The input amount of phosphorus trichloride in the present embodiment is 1037kg, methyl alcohol 483kg, sodium hydroxide 603kg, sulfur 242kg, the product O produced, the product amount of O-dimethylphosphoryl thiochloride is 1000kg. The total yield to pho...
Embodiment 2
[0057] Embodiment 2: The used raw material of this product synthesis is phosphorus trichloride, dehydrated alcohol, sulfur. First use phosphorus trichloride and absolute ethanol to react in a trialkyl synthesis kettle in the presence of solvent benzene and acid-binding agent triethylamine to generate triethyl phosphite. Triethyl phosphite reacts with phosphorus trichloride in the chlorinator to generate O, O-diethyl phosphorus monochloride, and then reacts O, O-diethyl phosphorus monochloride with sulfur in the vulcanizer Produce O, O-diethylthiophosphoryl chloride crude product; after post-treatment, O, O-diethylthiophosphoryl chloride is obtained.
[0058] The input amount of phosphorus trichloride in the present embodiment is 770kg, ethanol 519kg, sodium hydroxide 472kg, sulfur 181kg, and the product O produced, O-diethylthiophosphoryl chloride is 1000kg, and this product is to phosphorus trichloride The total yield is 94%, and its content is 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com