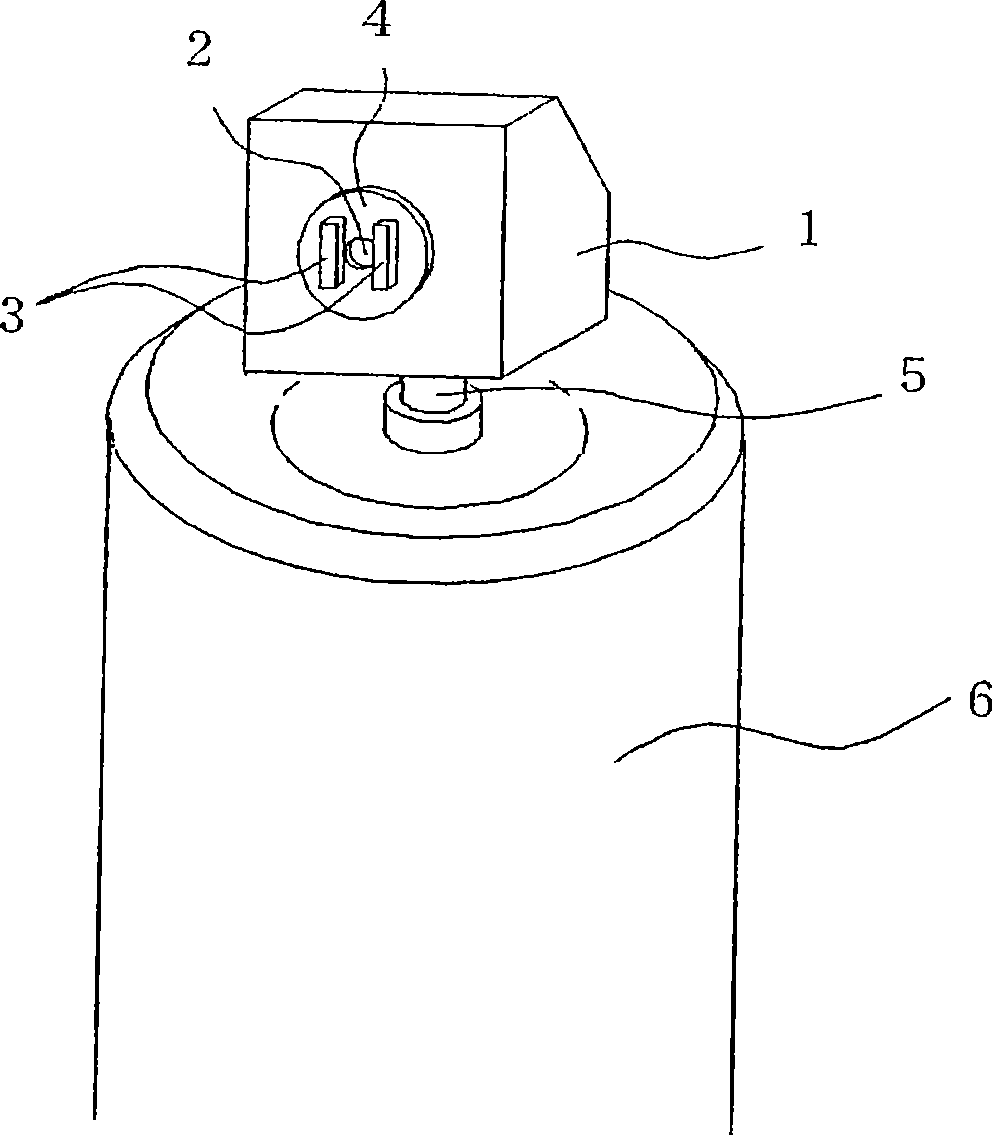
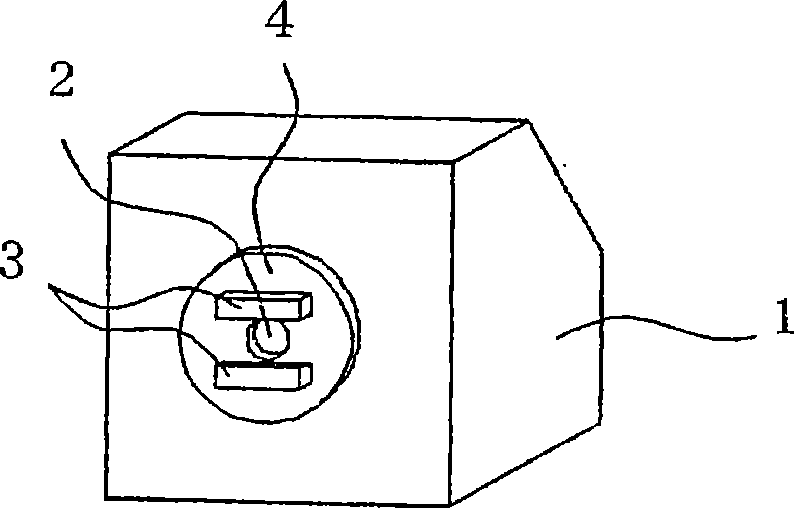
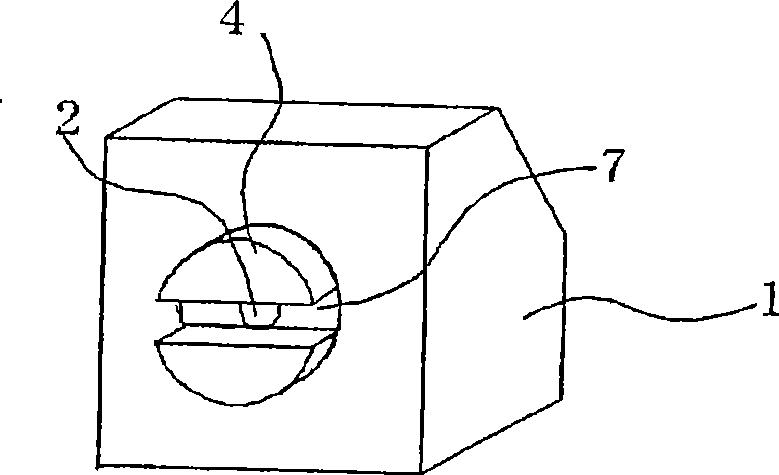
Aerosol of percutaneous medicament
A technology for aerosols and medicines, applied in the field of aerosols, can solve problems such as insufficient liquid flow, and achieve the effects of good use feeling, inhibition of liquid flow, and excellent efficacy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
manufacture example 1
[0075] Production Example 1 The stock solution was produced by the following method
[0076] (1) Add 0.5 g of hydroxypropylmethylcellulose 2906 (Metolose 65SH-1500: Shin-Etsu Chemical Industry) and 0.1 g of hydroxypropylcellulose (HPC-SSL: Nippon Soda) to 30 g of water at 80°C to make Cool and swell after dispersion to obtain an aqueous phase.
[0077] (2) Mix 6 g of propylene glycol, 7 g of polyethylene glycol, 1.8 g of benzyl alcohol, 4 g of diisopropyl adipate, and 36 g of isopropanol to obtain a uniform solution. Next, 3 g of 1-menthol and 1 g of indomethacin were dissolved in the obtained homogeneous solution to obtain a main drug phase.
[0078] (3) Mix and stir the aqueous phase obtained in (1) and the main drug phase obtained in (2), add the aqueous solution obtained by dissolving 0.01g sodium edetate in 5g water to the obtained liquid, and then add An appropriate amount of 1.2% by mass of diisopropanolamine aqueous solution was stirred well to prepare 100 g of a sto...
Embodiment 1
[0080] 65 parts by mass of the undiluted solution obtained in Production Example 1 and 35 parts by mass of dimethicone were airtightly filled in a device (nozzle in the device: F-96 (manufactured by Maruichi Co., Ltd.)) that exhibits a spray pattern that expands flat in one direction. Ether, obtains the aerosol of embodiment 1. In addition, when the spray particle diameter of the aerosol of Example 1 was measured with a laser diffraction particle size distribution analyzer (SPRAYTEC: manufactured by Malvern Co., Ltd.), it was 110 μm.
manufacture example 2
[0104] (1) Add 0.5 g of methyl cellulose (Metolose SM-4000: Shin-Etsu Chemical Industry) and 0.1 g of hydroxypropyl cellulose (HPC-SSL: Nippon Soda) to 30 g of water at 80°C, disperse and cool Swells to give an aqueous phase.
[0105] (2) Mix 6 g of propylene glycol, 7 g of polyethylene glycol, 1.8 g of benzyl alcohol, 4 g of diisopropyl adipate, and 36 g of isopropanol to obtain a uniform solution. Next, 5 g of 1-menthol, 2 g of methyl salicylate, 1 g of ethylene glycol salicylate, 2 g of dl-camphor, 0.5 g of eucalyptus oil, and 0.1 g of glycyrrhizic acid were dissolved in the obtained homogeneous solution to obtain a main drug phase.
[0106] (3) Mix and stir the aqueous phase obtained in (1) and the main drug phase obtained in (2), add the aqueous solution obtained by dissolving 0.01g sodium edetate in 5g water to the obtained liquid, and then add An appropriate amount of 1.2% by mass of diisopropanolamine aqueous solution was stirred well to prepare 100 g of a stock solut...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap