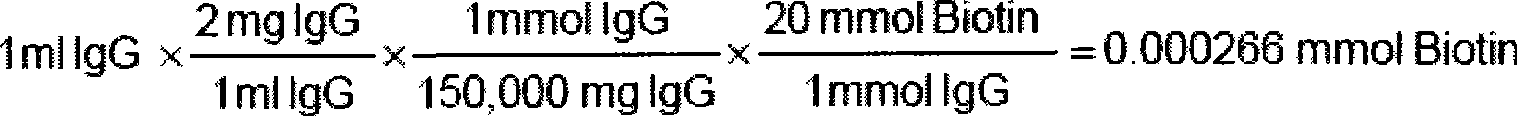Protein suspending chip for composite detection of multiple kinds of pathogens, its production method and detection method
A suspension chip and composite detection technology, which is applied in the direction of measuring devices, biological testing, material inspection products, etc., can solve the problems of lack of models and evaluations, and achieve the effects of simple preparation methods, wide dynamic range, and good applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] 2. Preparation of samples to be tested
[0045] 1. Preparation of samples for single-component analysis
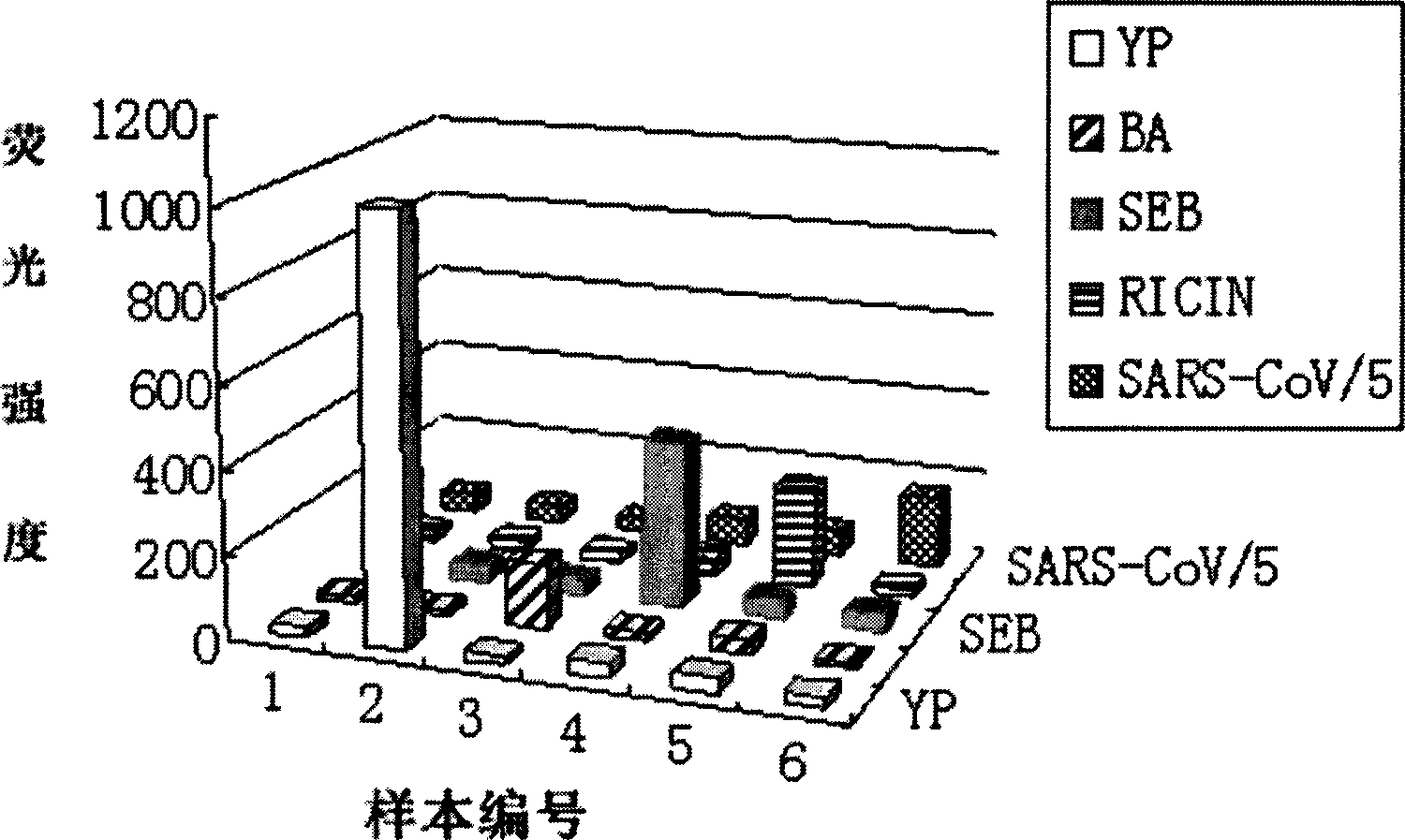
[0046] The concentration of Yersinia pestis stock solution was 10 8 cfu / mL, the concentration of anthrax spore stock solution is 10 7 cfu / mL, the stock solutions of ricin, SEB, and SARS-CoV N protein were all at 1 mg / mL. Toxin and protein samples were diluted just before use. The concentration range of the bacterial suspension was 10 1 -10 8 cfu / mL, the concentration range of spores is 10 2 -10 7 cfu / mL, toxin and protein concentrations ranged from 10 pg / mL to 5 μg / mL. The bacteria to be analyzed are diluted with PB into 10-fold different gradients, and the toxins and proteins are diluted with PB into 4-fold different gradients. The concentration of several samples is lower than the sensitivity of the detection. High-concentration samples should make the binding site of the encoded microspheres in the saturation state.
[0047] 2. Preparation of Mixed Sampl...
Embodiment 1
[0053] Embodiment 1, the preparation of protein suspension chip
[0054] A. Activation of encoded microspheres
[0055] Select 5 kinds of microspheres to label Yersinia pestis antibody (No. 028), anthrax spore antibody (No. 025), SEB antibody (043), ricin antibody (027), SARS-CoV N protein antibody (No. 044), and take 100 μL (1.25×10 6 pcs) encoded microspheres into a 1.5mL centrifuge tube, centrifuge at 14000g, carefully aspirate and discard the supernatant. Add 100 μL of microsphere washing buffer to suspend, shake and sonicate, centrifuge at 14000g, carefully aspirate and discard the supernatant. Add 100 μL of microsphere activation buffer, then add 10 μL of freshly prepared EDC (50 mg / mL), then add 10 μL of freshly prepared 50 mg / mL carboxyl-active biotin (ie Sulfo-NHS-biotin, SH-active biotin) and shake at room temperature for 20 minutes. Add 150 μL of PBS (pH7.4), shake, centrifuge at 14000 g, carefully aspirate and discard the supernatant. Add 100 μL of PBS (pH 7.4...
Embodiment 2
[0067] Embodiment 2, the improvement of the sensitivity of target pathogen and dynamic detection range
[0068] A. Preparation of samples to be tested
[0069] Yersinia pestis stock solution (10 8 cfu / mL) and anthrax spore stock solution (10 7 cfu / mL) was diluted 10 times with PB to form a series of concentration gradient samples, and ricin, SEB, and SARS-CoVN protein (stock solution 1 mg / mL) were diluted 4 times with PB to form a series of concentration gradient samples. The concentration range of Yersinia pestis suspension is 10 1 ~10 8 cfu / mL, the concentration range of anthrax spores is 10 2 ~10 7 cfu / mL, the concentrations of ricin, SEB, and SARS-CoV N protein ranged from 10 pg / mL to 5 μg / mL.
[0070] B. Testing of samples
[0071] All reactions in the detection process were carried out on a 96-well filter plate, and the detection process was as follows:
[0072] (1) Add 50 μL of working solution containing corresponding coded microspheres to each well, wash with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com