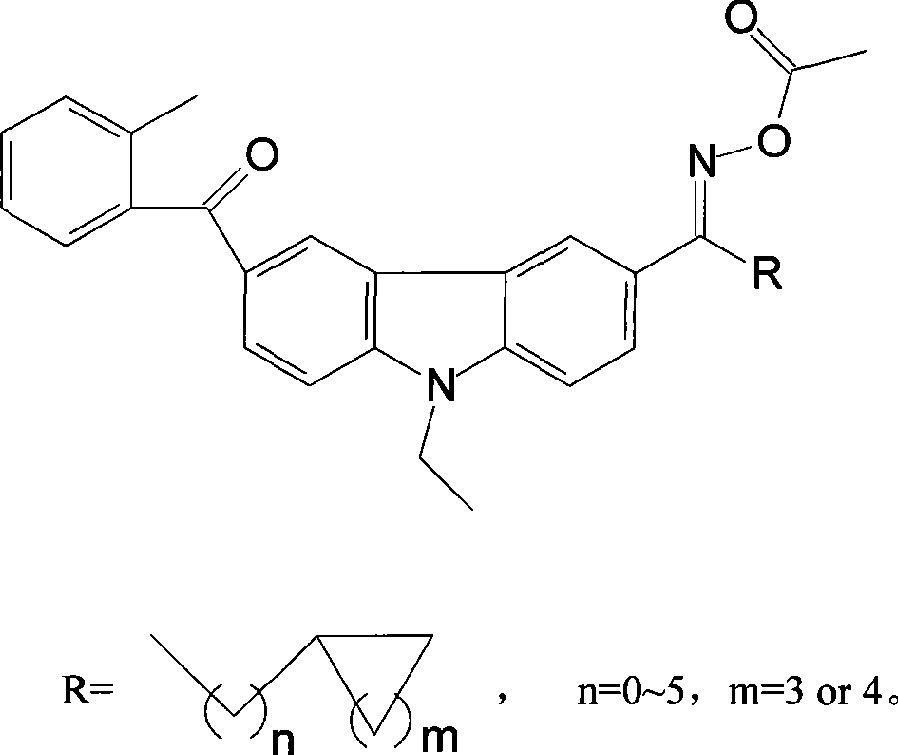
Carbazole oxime ester lightlike initiating agent
A technology of photoinitiator and carbazole oxime, which is applied in the field of oxime ester photoinitiator and its preparation, can solve the problems of poor sensitivity, thermal stability and solubility, and achieves low production cost, good application performance and product purity. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
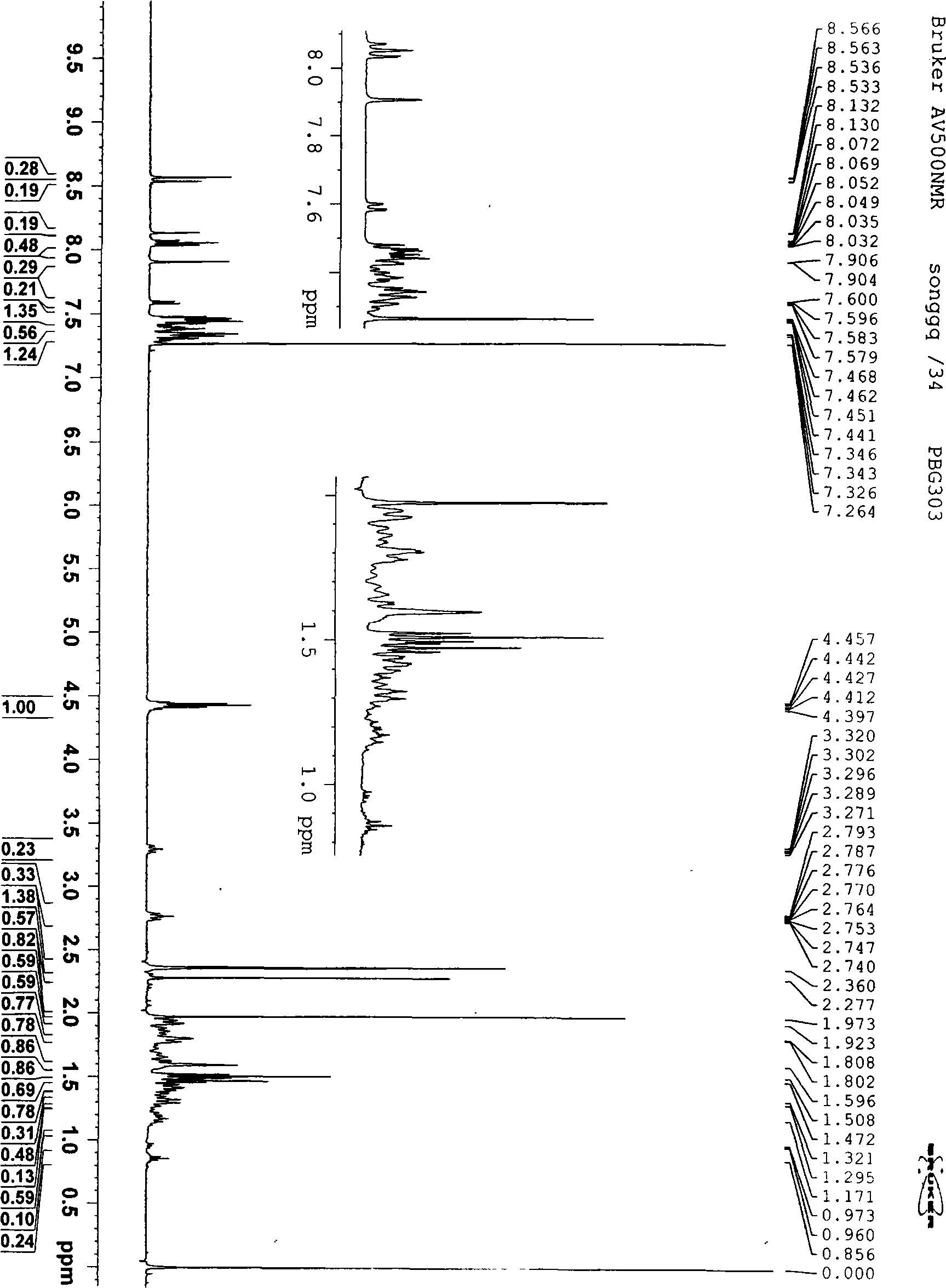
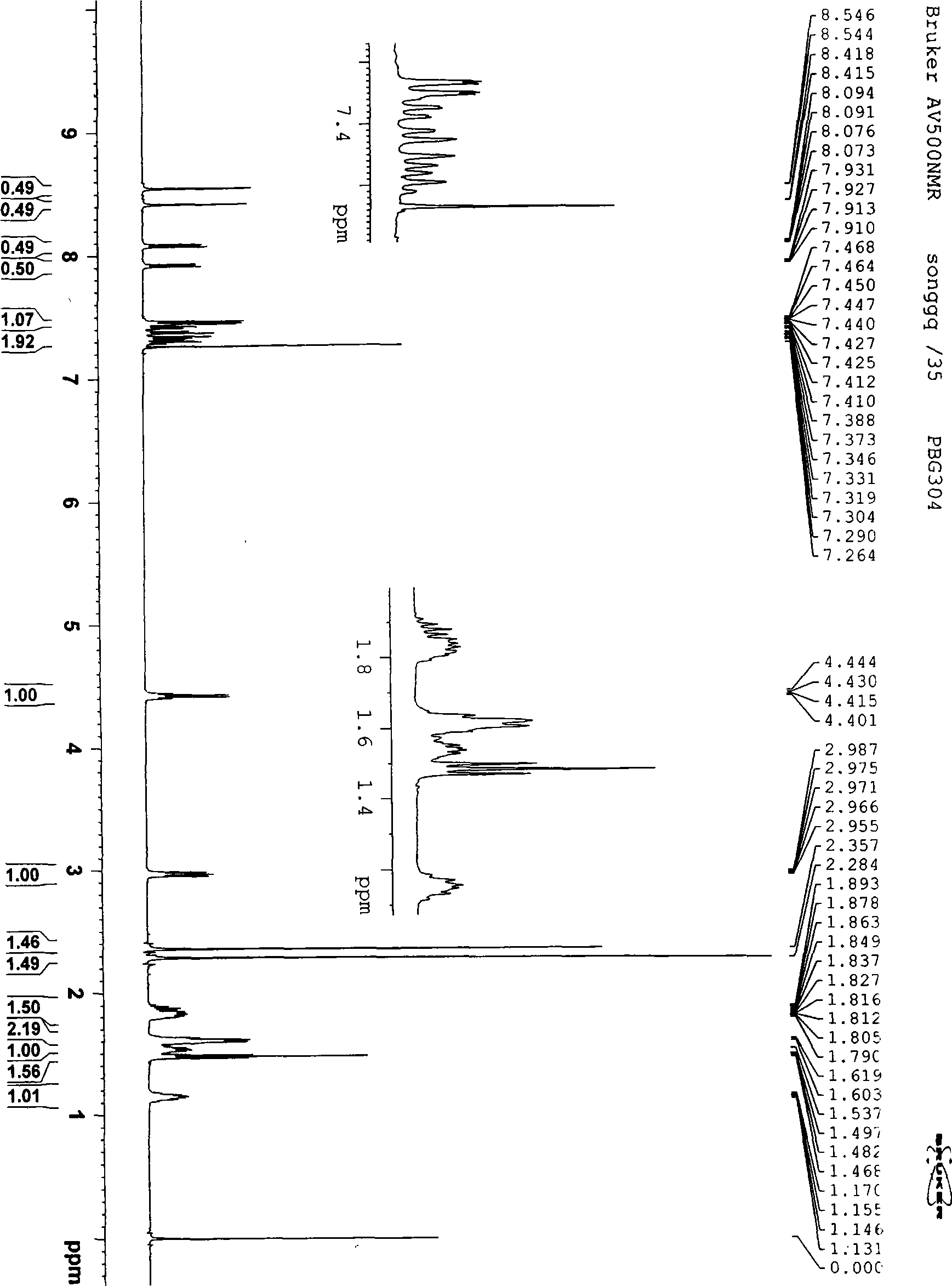
Image
Examples
Embodiment 1
[0031] The preparation method of 1-(6-o-methylbenzoyl-9-ethylcarbazole)-1-cyclohexyl ketone oxime ethyl ester
[0032] Step 1: Preparation of 3-(3-cyclohexyl)-6-o-methylbenzoyl-9-ethylcarbazole
[0033] Drop into 39.0g N-ethylcarbazole, 25.3g AlCl in the 500ml four-necked flask 3 (grind), 150ml of dichloromethane, stir, pass through argon protection, and cool in an ice bath. When the temperature drops to 0°C, start to drop 25.4g of o-toluyl chloride and 21g of dichloromethane's o-methyl Benzoyl chloride solution, the temperature is controlled below 10°C, the addition is completed in about 1.5h, and the stirring is continued for 2h, then 25.4g of AlCl is added to the flask 3 (Grind finely), add dropwise 29.3g of cyclohexanecarbonyl chloride and 20g of dichloromethane cyclohexanecarbonyl chloride solution, control the temperature below 10°C, drop it in about 1.5h, then raise the temperature to 15°C, continue to stir for 2h, and discharge .
[0034] Post-processing:
[0035] ...
Embodiment 2
[0045] Step 1: Preparation of 3-(3-cyclopentapropionyl)-6-o-methylbenzoyl-9-ethylcarbazole
[0046] Drop into 39.0g N-ethylcarbazole, 25.3g AlCl in the 500ml four-necked flask 3 (grind), 150ml of dichloroethane, stir, pass into argon protection, and cool in an ice bath. When the temperature drops to 0°C, start to drop 25.4g of o-toluyl chloride and 21g of dichloroethane. Toluyl chloride solution, the temperature is controlled below 10°C, the addition is completed in about 1.5h, and the stirring is continued for 2h, then 25.4g of AlCl is added to the flask 3 (grind finely), dropwise add 42.2g of cyclopentanoyl chloride and 20g of dichloroethane cyclohexanecarbonyl chloride solution, keep the temperature below 10°C, drop it in about 1.5h, then raise the temperature to 15°C, continue to stir for 2h, and material.
[0047] Post-processing:
[0048] Under stirring, slowly pour the material into dilute hydrochloric acid made of 400g ice and 65ml concentrated hydrochloric acid, se...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com