Recombinant carboxyl peptidase G2 expression vector and method for preparing recombinant carboxyl peptidase G2
An expression vector and carboxypeptidase technology, applied in the biological field, to achieve the effects of high product yield, low production cost, and simple culture medium
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Example 1 Construction of Escherichia coli Expression Vector of Recombinant Carboxypeptidase G2
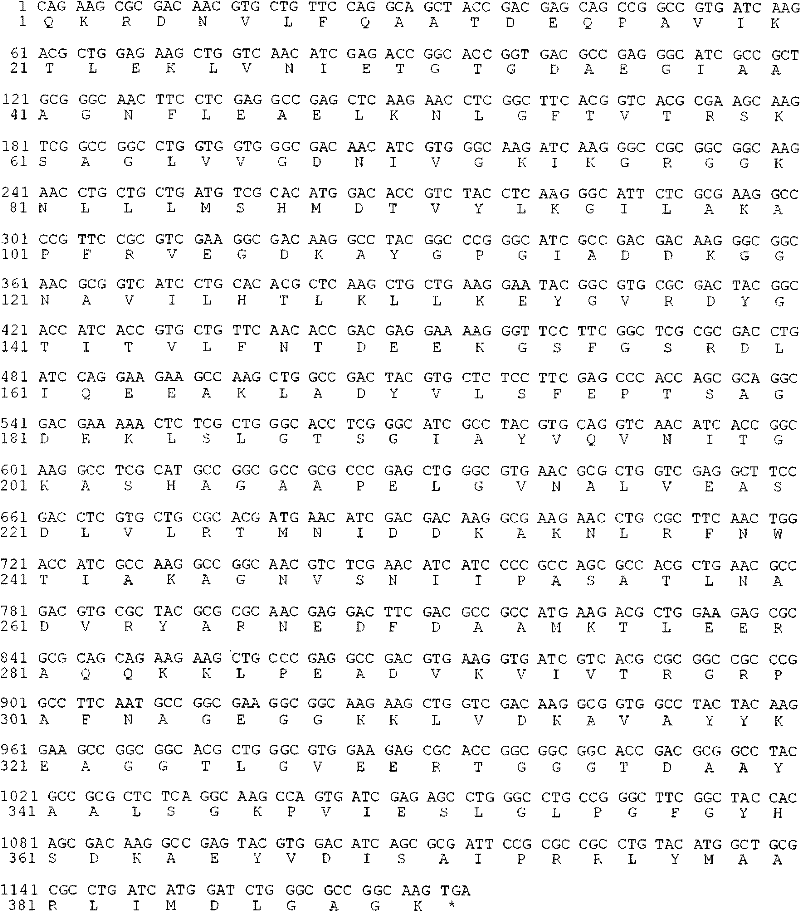
[0068] 1. Gene synthesis
[0069] According to the CPG2 gene sequence (accession number: M12599) registered in GenBnak, the whole gene synthesis (completed by Shanghai Sangong), the sequence is shown in figure 2 .
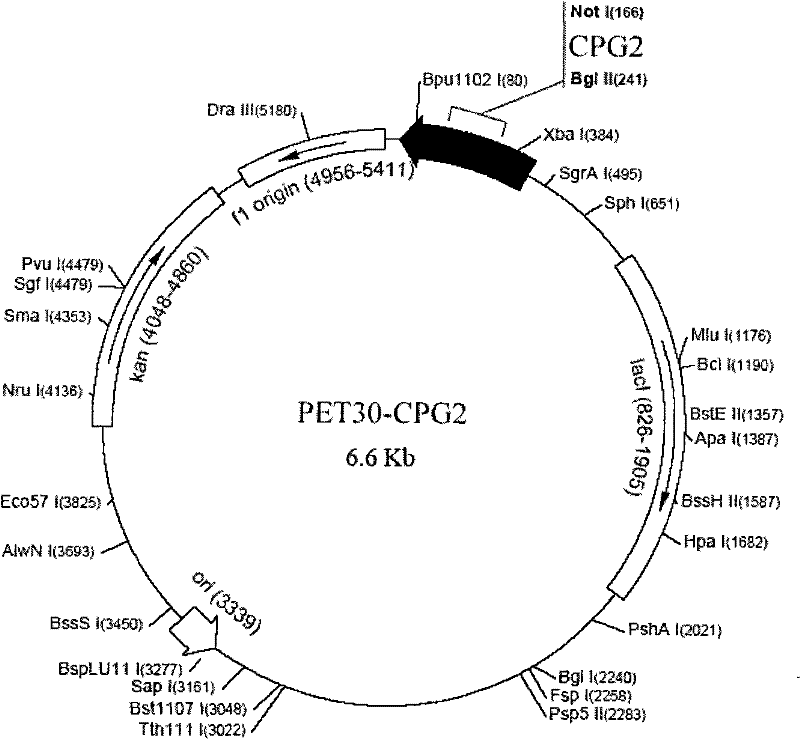
[0070] 2. Construction of PET30-CPG2 expression vector
[0071] 2.1PCR amplification
[0072] Using the synthetic CPG2 gene as a template, PCR amplification was performed with primer 1 and primer 2 and high-fidelity Taq enzyme. Primer 1 introduced a BglII restriction site and an enterokinase restriction site, and primer 2 introduced a NotI restriction site. The PCR amplification parameters were: pre-denaturation at 94°C for 5 minutes, followed by 35 cycles (94°C for 30 seconds, 50°C for 30 seconds, 72°C for 1.5 minutes), and 72°C for 10 minutes to end the reaction.
[0073] Primer 1:
[0074] 5'>TGA AGATCT G GACGACGACGACAAG CAGAAGCGCGACAACGTG<3
[...
Embodiment 2
[0083] Example 2 Construction of engineering strains expressing recombinant carboxypeptidase G2 in Escherichia coli
[0084] 1. Preparation of BL21(DE3) and BL21(DE3)pLysS Competent Cells
[0085] Adopt calcium chloride method to prepare Escherichia coli BL21 (DE3) competent cell, its method is as follows:
[0086] Pick a single colony of BL21(DE3) in 25ml LB liquid medium, culture overnight at 37°C, 250r / min; inoculate 1ml of the overnight culture solution in 100ml LB the next day, and cultivate to OD at 37°C, 250r / min 600 0.375, about 2.5 hours; divide the culture solution into pre-cooled sterile polypropylene tubes, place on ice for 5-10 minutes, and then centrifuge at 4°C, 2000r / min for 7 minutes; discard the supernatant, and use 10ml ice-cold CaCl 2 Resuspend the bacterial pellet in the solution, centrifuge at 1600r / min for 5 minutes at 4°C; discard the supernatant, and wash with 10ml ice-cold CaCl 2 The solution resuspends the cell pellet, and places it on ice overn...
Embodiment 3
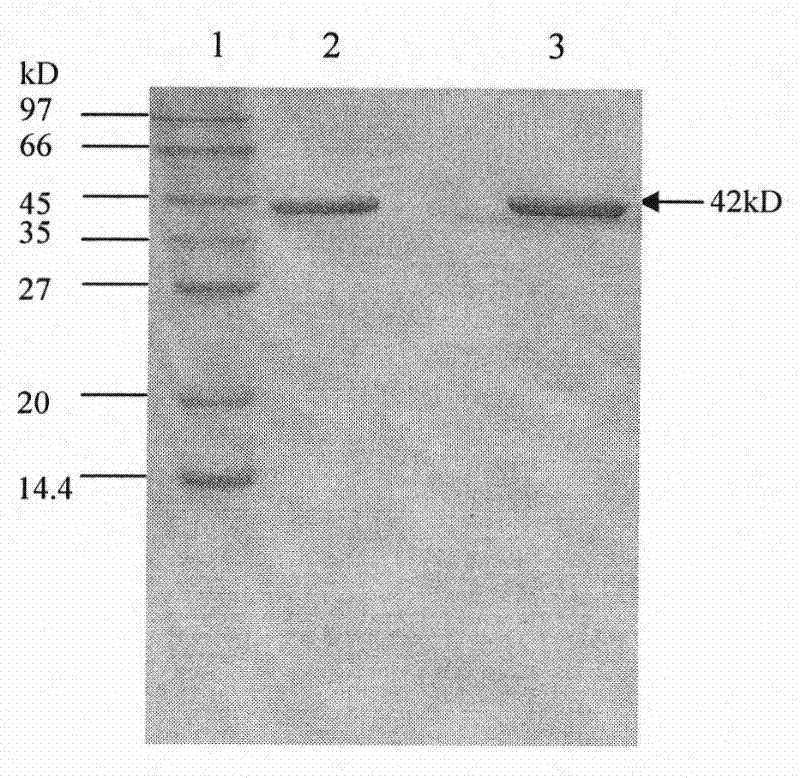
[0093] Example 3 Expression and Purification of Recombinant Carboxypeptidase G2
[0094] Pick engineering bacteria PET30-CPG2 / BL21 (DE3) and inoculate into 20ml LB (Kan 50ug / mL) liquid medium, cultivate overnight at 37°C and 250rpm. The next day, inoculate the mother liquor into 200ml LB (Kan50ug / mL) liquid medium at a ratio of 1%, and culture it in a 1L Erlenmeyer flask at 37°C and 250rpm until the OD 600 After reaching 0.6, add 100mM IPTG to a final concentration of 0.4mM, and continue to induce expression at 30°C for 2-3 hours. Place the shake flask on ice for 5 minutes, centrifuge at 5000g, 4°C for 5 minutes to collect the bacteria.
[0095] The bacteria in the fermentation broth were suspended in the lysate (20mM Tirs-HCl, 0.2mM Zn 2+ , pH8.0), sonicated the wall, and centrifuged at 8,000rpm for 5min to collect the supernatant; the supernatant of the bacteriostasis solution was loaded on NiSO4 treated with 0.2M NiSO4 and equilibrated with 20mM Tris-HCl (pH8.0) 2+ -Ch...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com