Self preserved aqueous pharmaceutical compositions
A technology of composition and ophthalmic composition, applied in the field of actic, can solve problems such as blindness and loss of visual function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A-E
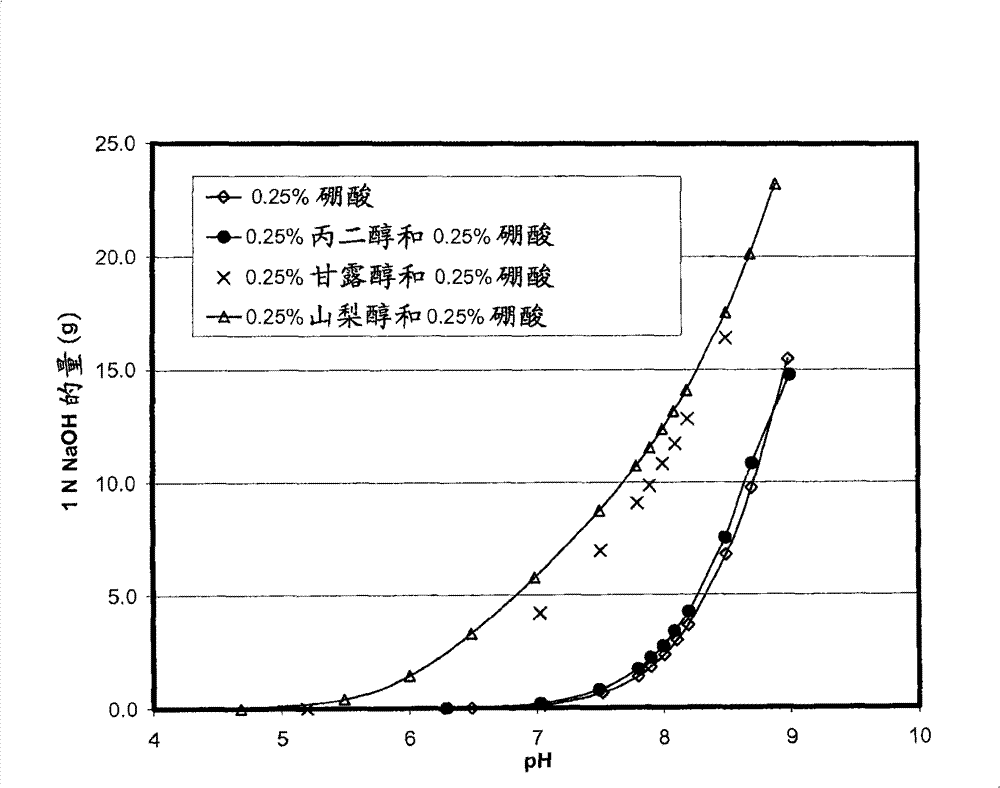
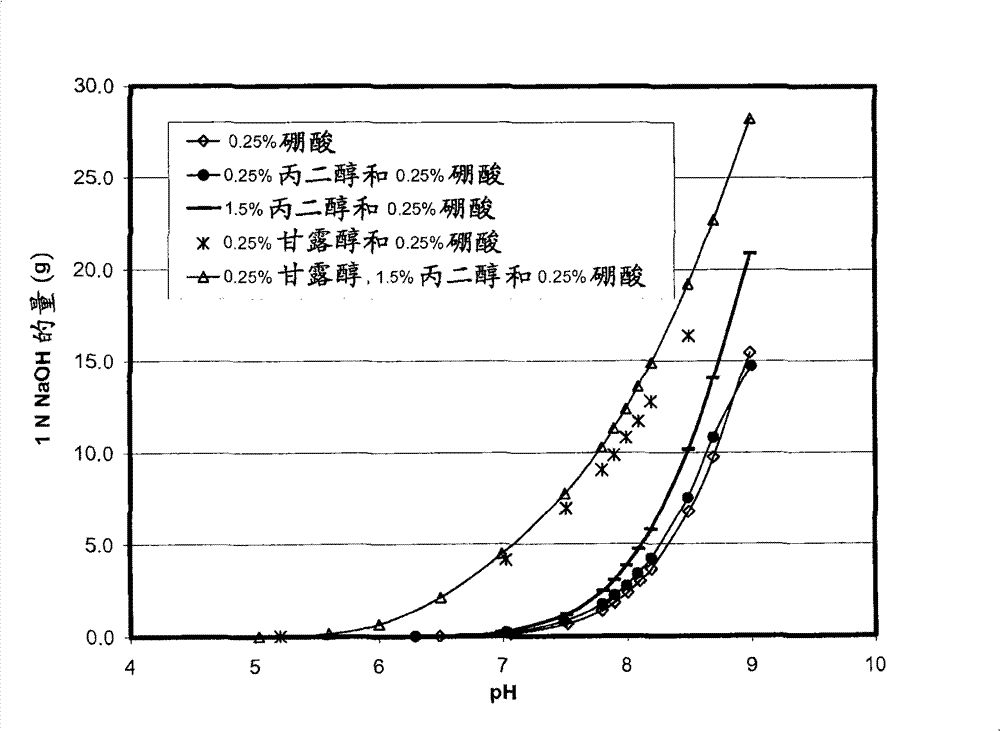
[0091] The formulations of Examples A-E were evaluated to determine the effect of buffer anions on preservative effectiveness. As detailed below, the formulations of Examples A and B do not contain buffers. Although these formulations meet the USP preservative effectiveness requirements, there is still a great need for a buffer system in order to prevent pH deviations within the shelf life of the commodity (ie, up to two years or more). The formulation of Example C contains a borate / polyol buffer system, but this system has the smallest buffer capacity. Like the formulations of Examples A and B, the formulation of Example C meets USP requirements. The formulations of Examples D and E contain a significantly higher concentration of buffer, and therefore have a greater buffer capacity. However, the presence of a relatively large amount of buffer anion causes the formulation to fail to meet the requirements for preservative efficacy. Therefore, the comparison of Examples A-E sh...
Embodiment F-J
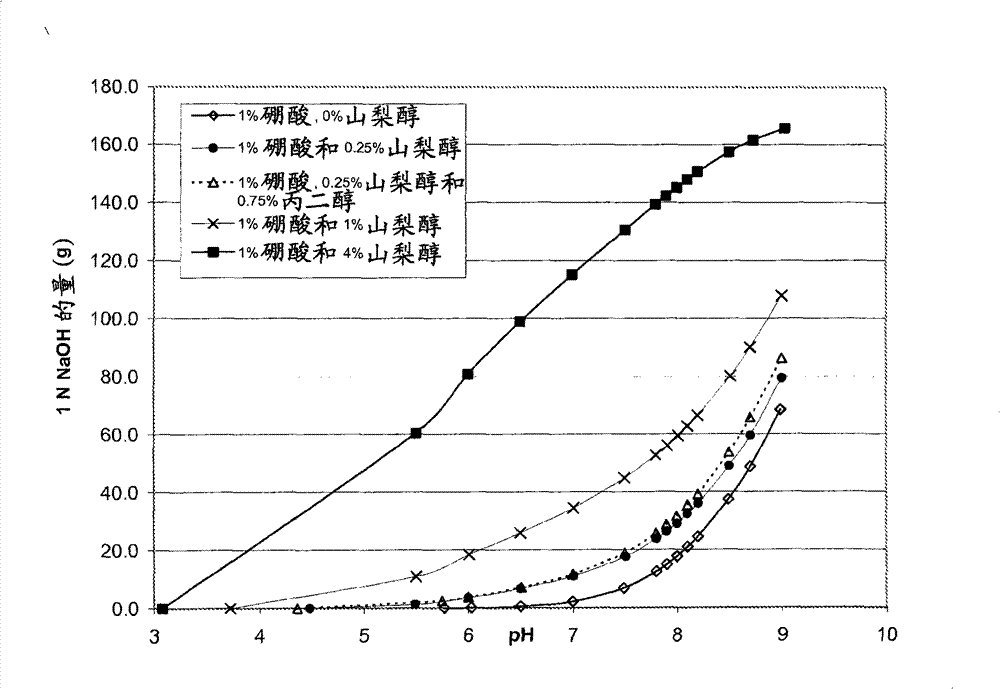
[0099] In these examples, the amount of sorbitol was reduced to 1% while maintaining the concentration of boric acid at 1% to reduce the concentration of buffer anions. In addition, Examples G, I and J contained 0.75% propylene glycol. The anion buffer concentration in all 5 examples was about 19 mM.
[0100] The compositions of Examples F and G contained 0.18 mM zinc. It has much higher activity against Staphylococcus aureus than the preparations of Examples D and E above. In particular, the compositions of Examples F and G meet the USP preservative standards for Staphylococcus aureus. However, although the antibacterial activity against E. coli is improved when the zinc ion concentration is 0.18 mM (Examples F and G) and 0.36 mM (Examples H and I) compared with Examples D and E, it is in the first 14 days is not enough to continuously meet the USP anti-corrosion standards. Increasing the zinc concentration to 1.8 mM (Example J) improved the antibacterial activity of the sol...
Embodiment K-N
[0106] In these examples, the content of sorbitol was reduced to 0.25% while maintaining the concentration of boric acid at 1% to reduce the concentration of buffer anions. In addition, the compositions of Examples L-N contained 0.75% of propylene glycol. The anion buffer concentration of the formulations of Examples K and L is about 4 mM, which is in the preferred range below 5 mM as described herein. Compared with the formulations of Examples F-J, the anti-E. coli activity of these compositions was significantly improved when the zinc concentration was 0.18 mM (0.0025 w / v%), and the compositions met the USP preservative standards. In Examples M and N, the pH was adjusted to 5.5 and 6.5, respectively, while maintaining the preservative effect of USP. The results obtained for the representative composition of the present invention, namely the formulations of Examples K-N, further demonstrate the importance of limiting the buffer anion concentration to meet the requirements for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com