Non-aqueous electrolyte secondary battery
A non-aqueous electrolyte and secondary battery technology, applied in secondary batteries, battery electrodes, circuits, etc., can solve the problems of graphite material capacity not necessarily sufficient, charge-discharge cycle characteristics and other battery characteristics reduction, discharge characteristics reduction, etc., to achieve Effects of preventing degradation of charge-discharge cycle characteristics, suppressing decomposition of non-aqueous electrolyte, and improving charge-discharge cycle characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
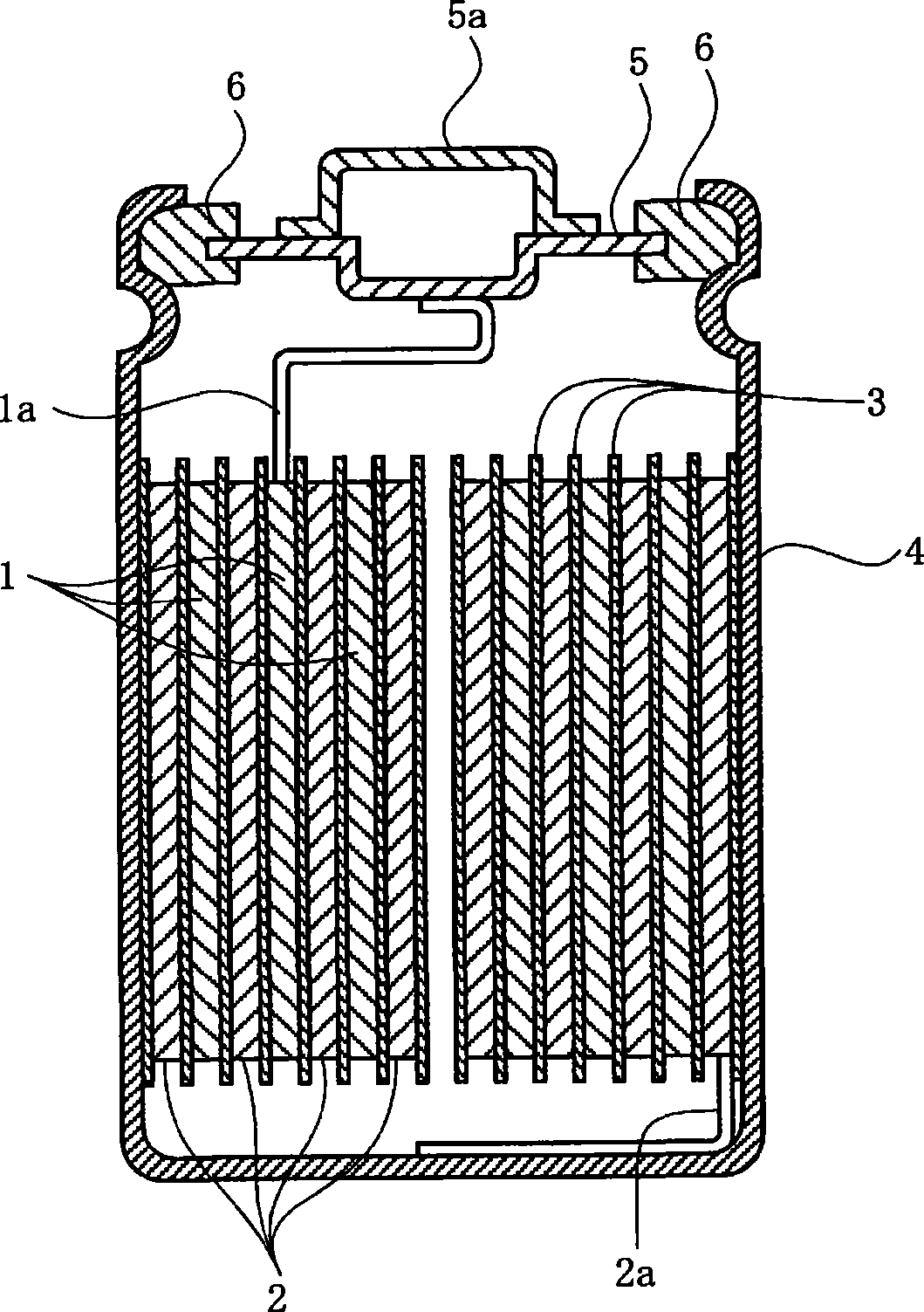
[0057] In Example 1, using the positive electrode, negative electrode, and non-aqueous electrolyte prepared as described below, a cylindrical shape with a diameter of 14 mm and a height of 43 mm with a design capacity of 950 mAh was manufactured. figure 1 The non-aqueous electrolyte secondary battery shown.
[0058] Production of positive electrode
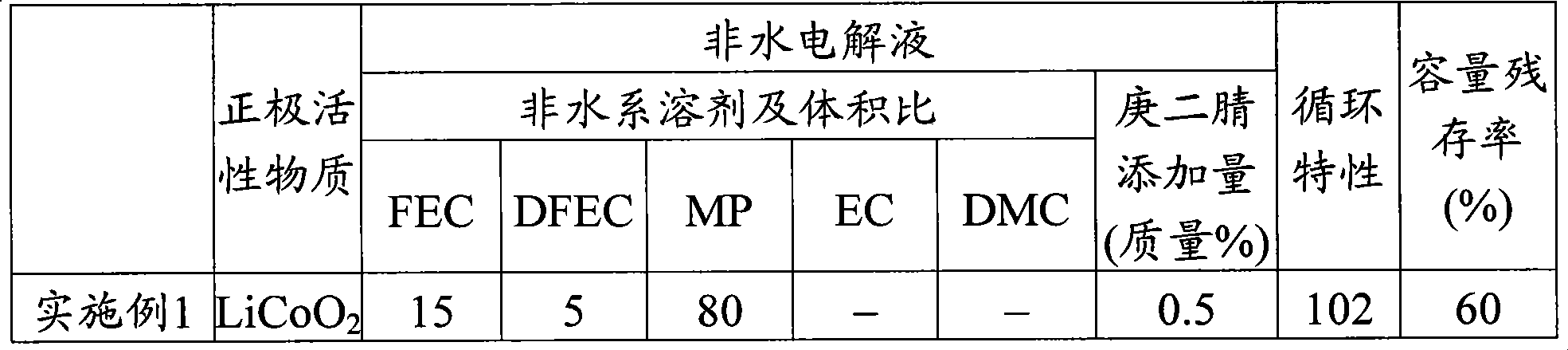
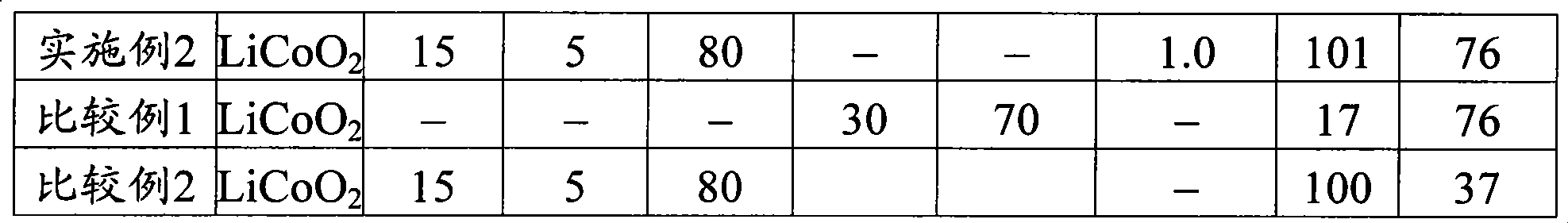
[0059] When making the positive electrode, use LiCoO 2 Said lithium cobalt oxide (average particle size 13μm, BET specific surface area 0.35m 2 / g) As the positive electrode active material, mix the positive electrode active material, conductive agent carbon material powder, and binder polyvinylidene fluoride in a mass ratio of 95:2.5:2.5, and mix the N-methyl-2-pyrrolidone solution It is added to this mixture and kneaded to prepare a positive electrode mixture slurry.
[0060] Then, the positive electrode mixture slurry was coated on both sides of a positive electrode current collector made of aluminum foil with a thickness of 15 ...
Embodiment 2
[0070] In Example 2, the non-aqueous electrolyte secondary battery of Example 2 was produced in the same manner as in Example 1, except that when the non-aqueous electrolyte was prepared in Example 1, the above-mentioned nitrile compound was added to the non-aqueous electrolyte. That is, the amount of pimelic nitrile is 1.0% by mass.
Embodiment 3
[0089] In Example 3, the non-aqueous electrolyte secondary battery of Example 3 was produced in the same manner as in Example 1, except that when the non-aqueous electrolyte was prepared in Example 1, succinonitrile was used as the non-aqueous electrolyte. The added amount of the added nitrile compound having a chain saturated hydrocarbon group with a carbon number of 2 or more is 1.0% by mass.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Filling density | aaaaa | aaaaa |
| Surface roughness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com