Conjugate conjugated from polyethylene glycol and curcumin derivative
A technology of curcumin derivatives and polyethylene glycol is applied in directions such as pharmaceutical combinations, medical preparations of non-active ingredients, active ingredients of ketones, etc., and can solve the problems of high cost, many preparation process steps, complex formula and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
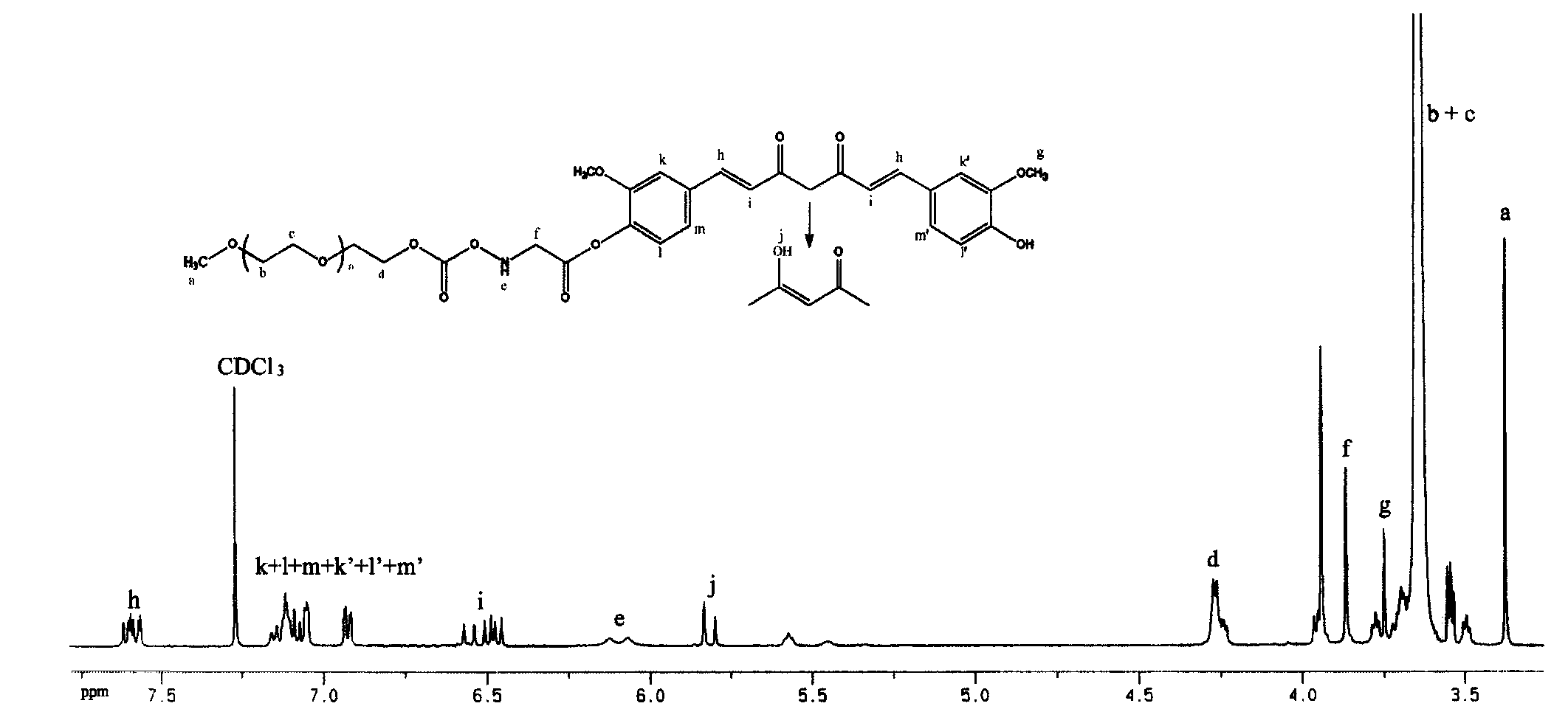
[0046] Embodiment 1: monomethoxy polyethylene glycol 2k -OCO-glycine-curcumin (mPEG 2K -OCO-Gly-Cur) synthesis
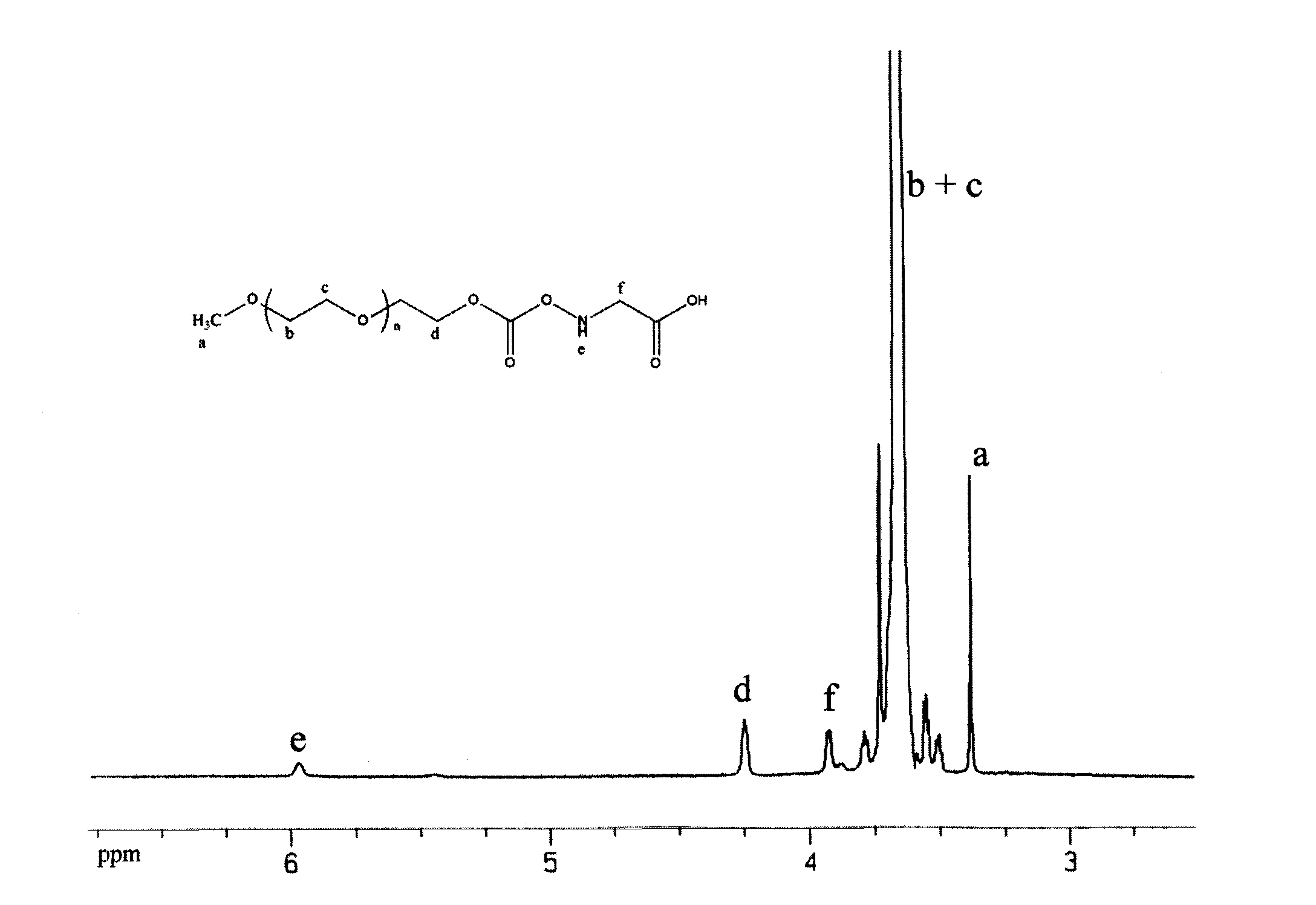
[0047] 1. mPEG 2K -Synthesis of pNC: Weigh mPEG 2K (purchased from Fluka Company) 20.0g (10.0mmol), add 100ml toluene to dissolve, evaporate under reduced pressure at 60°C; add 200ml temporarily dried CH 2 Cl 2 Dissolve, then weigh 6.465g (30.0mmol) of pNPC and add it to the above system, stir and add 6ml of temporary dry Py, stir and react at room temperature (about 20°C) in the dark for about 12h; Crystallization for 2 hours; suction filtration, rinsing with anhydrous ether, recrystallization once with anhydrous ether, vacuum-dried white product 20.85g, yield 96.32%.
[0048] Accurately weigh an appropriate amount of the above product, dissolve it with 0.1M NaOH, and measure the absorbance value at 402nm, according to the molar absorptivity of 18400cm -1 m -1 The amount of p-nitrophenol released was calculated, and the purity of the product was es...
Embodiment 2
[0054] Embodiment 2: monomethoxy polyethylene glycol 5k -OCO-glycine-curcumin (mPEG 5K -OCO-Gly-Cur) synthesis
[0055] Its preparation method is basically the same as Example 1, and the main differences of each step are:
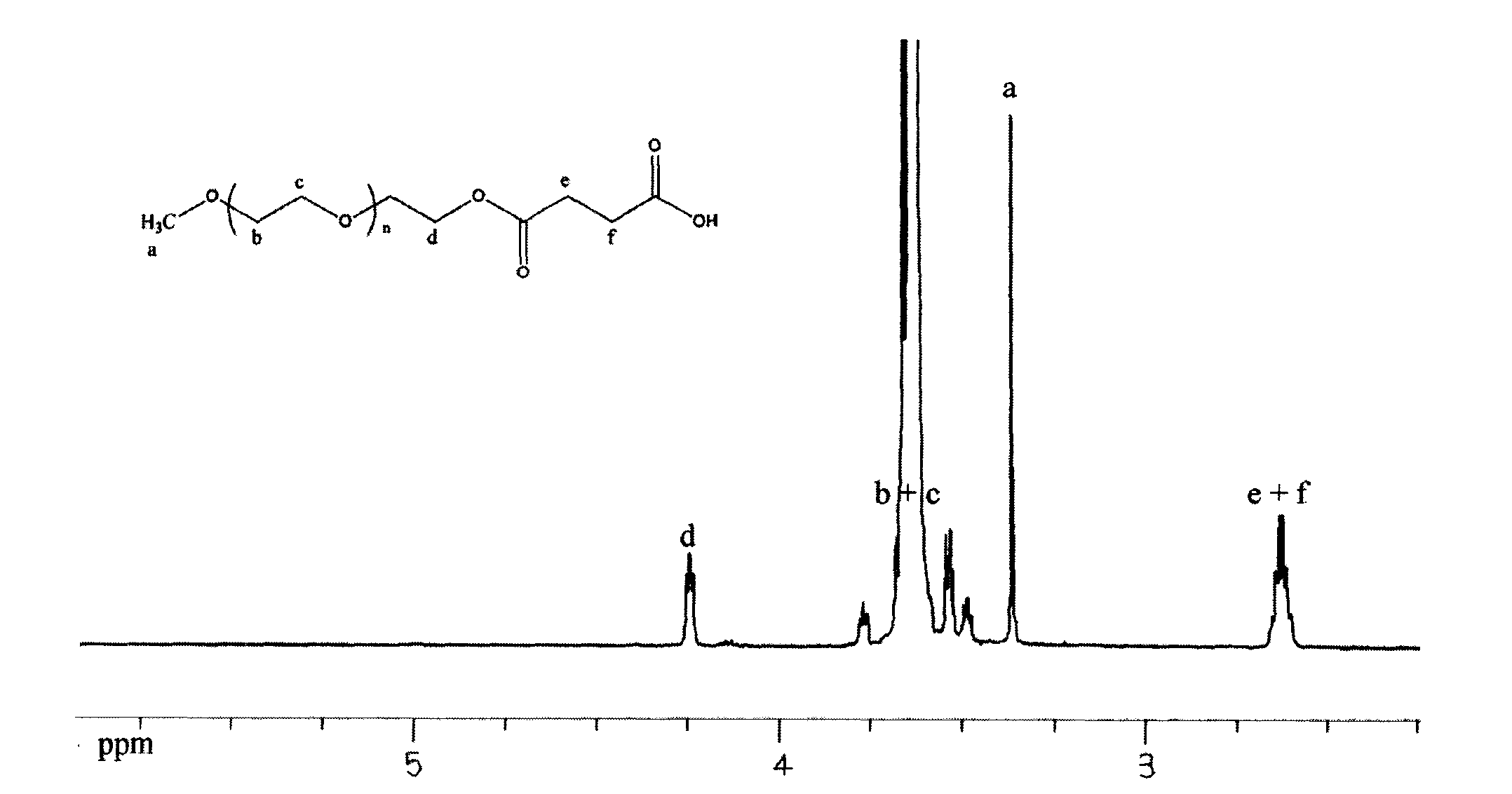
[0056] 1. mPEG 5K -Synthesis of pNC: Weigh mPEG 5K (purchased from Fluka Company) 40.0g (8.0mmol), add about 200ml of toluene to dissolve, evaporate under reduced pressure at 60°C; add 300ml of temporarily dried CH 2 Cl 2 Dissolved, then weighed 7.257g (36.0mmol) of pNPC and added to the above system, stirred and added 6ml of temporary dry Py, and stirred at room temperature in the dark for 12h; the product was 39.86g, the yield was 96.47%, and the purity was greater than 95.64%.
[0057] 2. mPEG 5K -OCO-Gly synthesis: Weigh glycine (hereinafter referred to as Gly) 2.402g (32.0mmol), dissolve with 150ml distilled water, add 100ml acetonitrile (H 2 O / CH 3 CN=3 / 2) and 20.66g (4.0mmol) mPEG 5k -pNC, the pH value of the system was adjusted to...
Embodiment 3
[0060] Embodiment 3: polyethylene glycol 6k -(OCO-Glycine-Curcumin) 2 [PEG 6K -(OCO-Gly-Cur) 2 ]Synthesis
[0061] The preparation method is basically the same as Example 1, and the main differences of each step are:
[0062] 1. PEG 6K -(pNC) 2 Synthesis: weigh PEG 6K (purchased from Fluka Company) 20.0g (3.33mmol), add 150ml toluene to dissolve, evaporate under reduced pressure at 60°C; add 350ml temporarily dried CH 2 Cl 2 Dissolve, then weigh 4.031g (20.00mmol) of pNPC and add it to the above system, stir and add 6ml of temporary dry Py, and stir at room temperature in the dark for about 12h; the product is 19.99g, the yield is 94.84%, and the purity is greater than 93.12%.
[0063] 2. PEG 6K -(OCO-Gly) 2 Synthesis: take glycine 2.40g (32.0mmol), dissolve with 150ml distilled water, add 100ml acetonitrile (H 2 O / CH 3 CN=3 / 2) and 12.66g (2.0mmol) PEG 6k -(pNC) 2 , the pH value of the system was adjusted at 8.5 with triethylamine, and the react...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com