Method for preparing phosphonyl methoxyl nucleotide analogue
A technology of phosphonomethoxy nucleotide and diethylphosphonomethoxy nucleotide is applied in the field of synthesizing phosphonomethoxy nucleotide analogs, and can solve the problems of high market price and high production cost of PMPA, To achieve the effect of easy control of process operation, low cost and safe reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
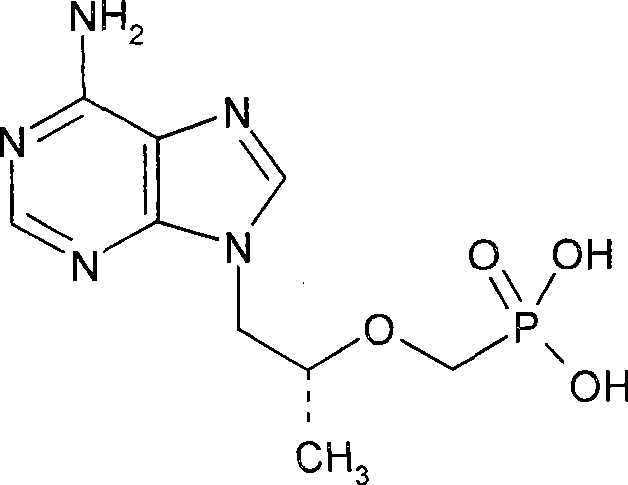
[0020] (R)-9-[2-(phosphonomethoxy)propyl]-adenine (PMPA)
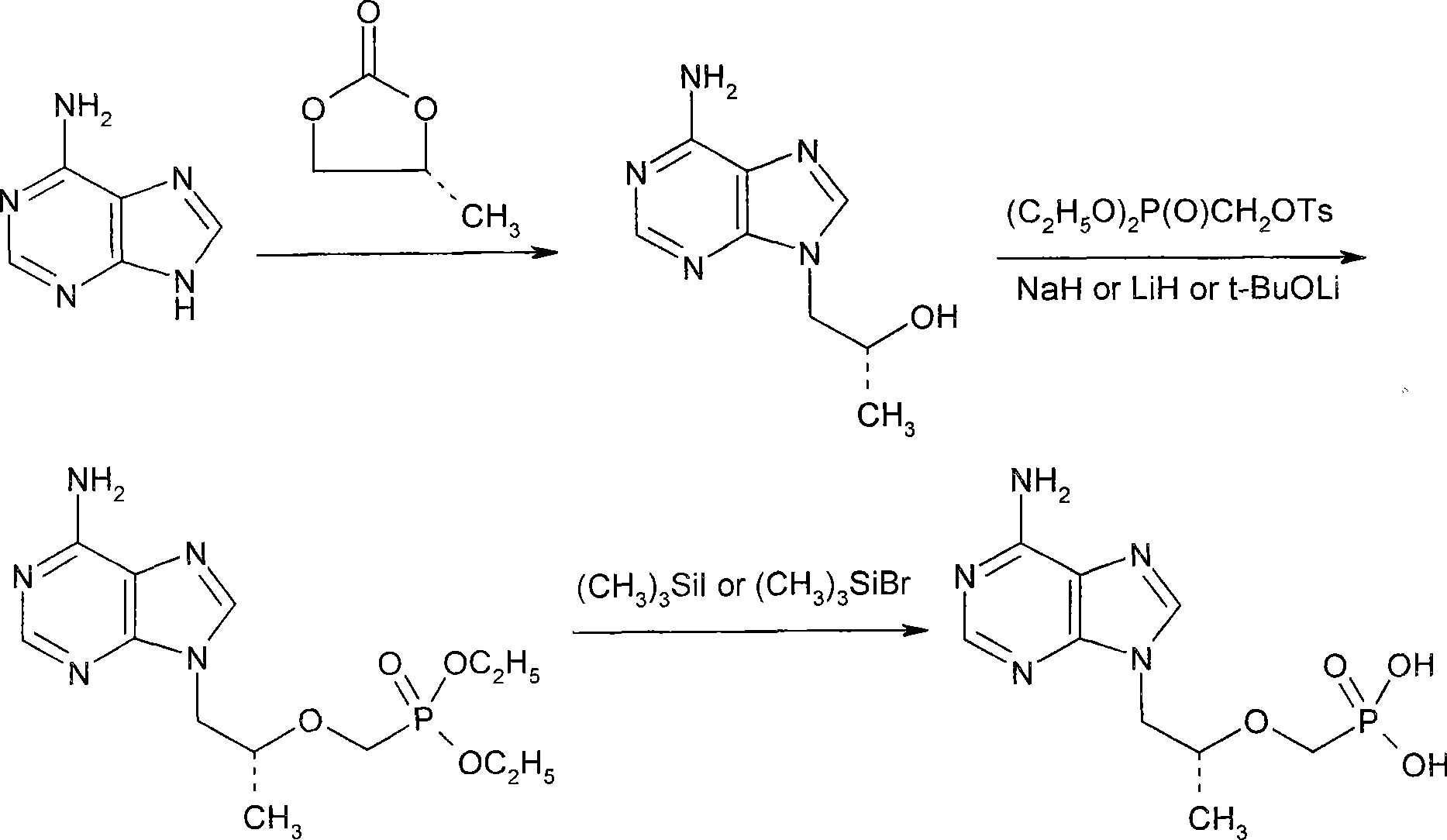
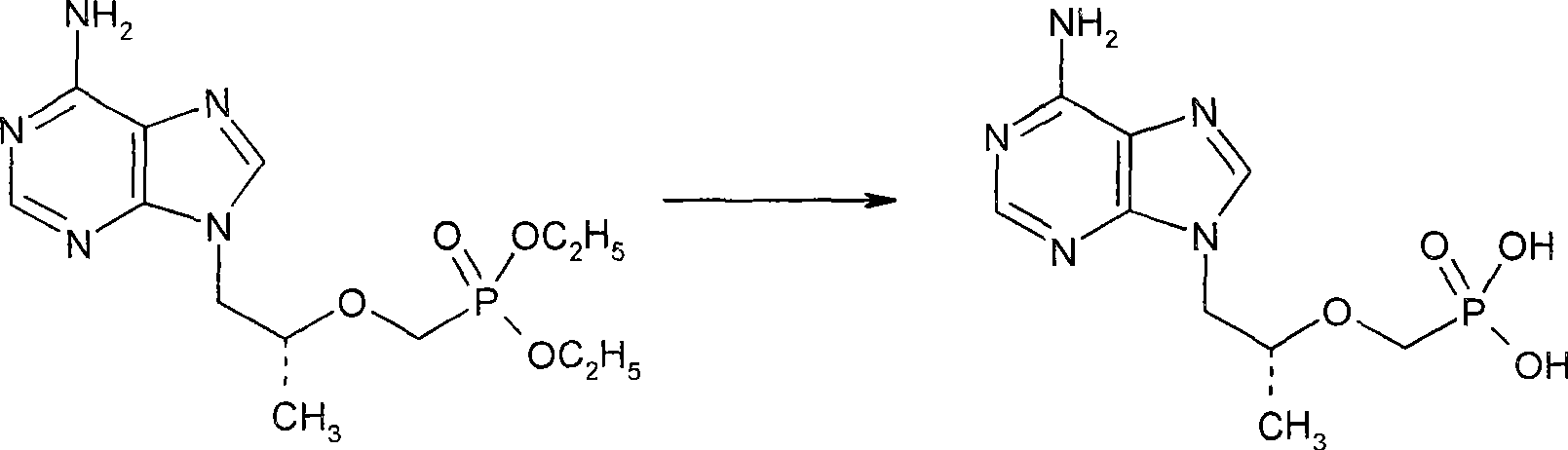
[0021] Dissolve 38.0g of PMPA diethyl ester in 38ml of acetonitrile, add 12.0g of sodium bromide, then control the system at 45°C, add 29.0g of trimethylchlorosilane dropwise, heat up to reflux after the addition, and reflux for 4 hours, TLC followed the reaction. The reaction was terminated after 3 hours, and evaporated to dryness under reduced pressure below 70°C. After cooling the residue to 20°C, 70ml of water was added to control the internal temperature not to exceed 50°C. Then cool to 20°C, add 60ml of dichloromethane and extract twice to remove less polar impurities. The separated aqueous layer was diluted with 50 ml of water and heated to 40°C. The pH meter measured about 1.1, and adjusted the pH value to 2.8 with 0.1N NaOH; then lowered the system temperature to 20°C, and solids began to precipitate after 0.5 hours. After a large amount of solids precipitated, adjust the pH value with 0.1N NaOH to 3.2, th...
Embodiment 2
[0024] (R)-9-[2-(phosphonomethoxy)propyl]-adenine (PMPA)
[0025] Dissolve 35.0g of PMPA diethyl ester in 38ml of acetonitrile, add 16.0g of sodium bromide, then control the system at 45°C, add 25.0g of trimethylchlorosilane dropwise, heat up to reflux after the addition, and reflux for 4 hours, TLC followed the reaction. Finish reaction after 4 hours, post-reaction treatment is basically the same as above, just add 0.2g PMPA obtained in Example 1 as seed crystal after adjusting the pH value to 2.8 with 0.1N NaOH, a large amount of solids are precipitated immediately after 5 minutes like this, other procedures are the same as above . 13.6g of the PMPA fine product obtained. It was analyzed by HPLC area normalization method, and the purity was 99.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com