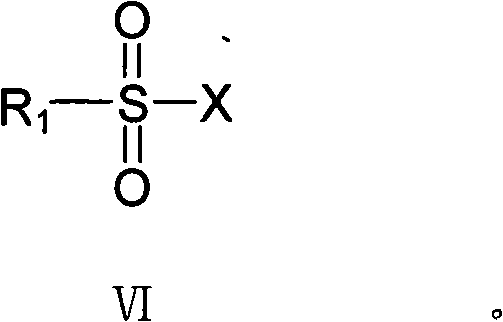Method for preparing citalopram and S-citalopram
A technology for citalopram and a compound is applied in the field of preparing citalopram and S-citalopram, which can solve the problems of high pressure on production and environmental protection, and achieve the effects of less three wastes, high yield and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
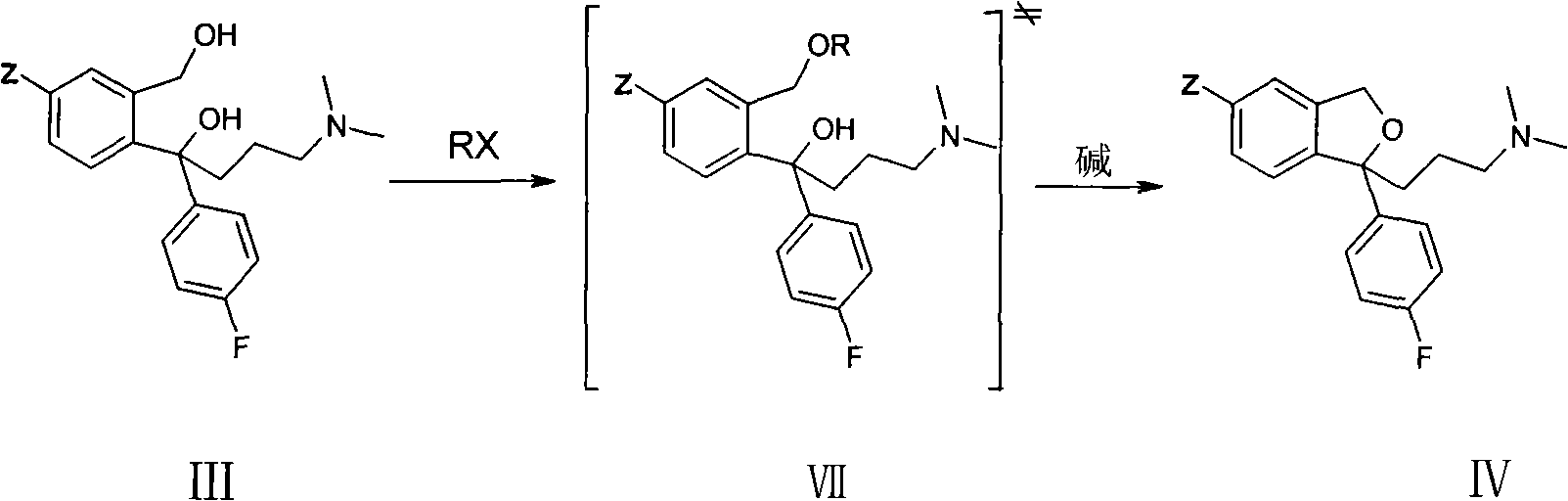
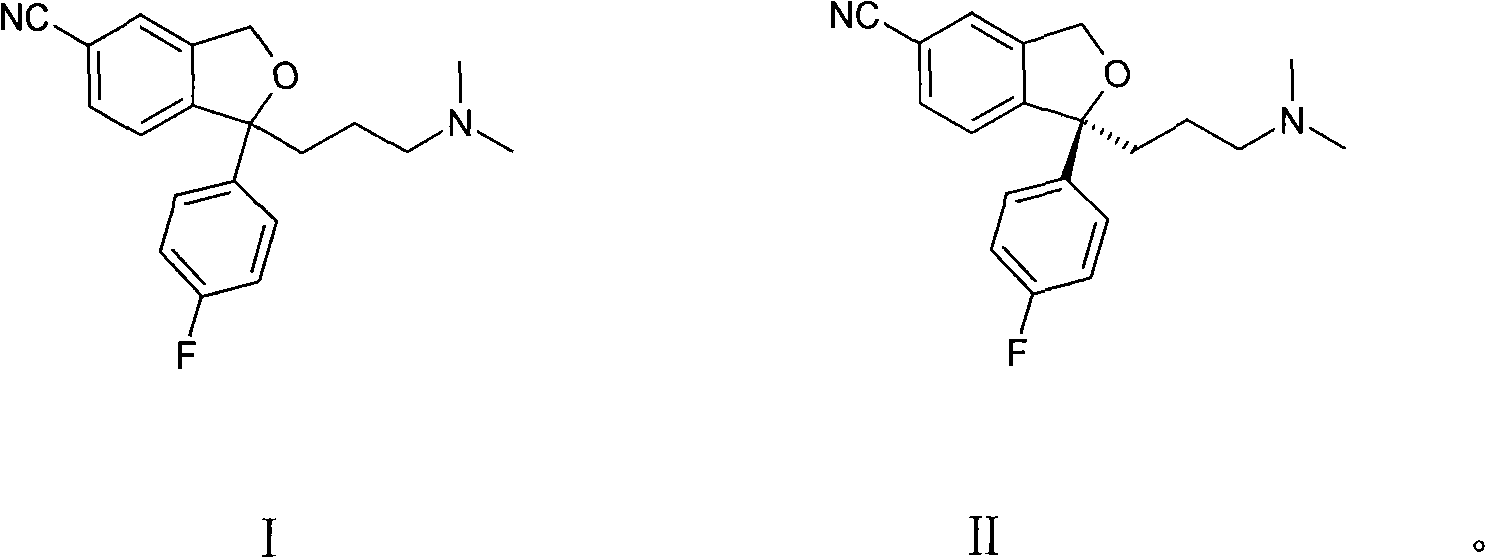
[0027] Example 1 (S)-(+)-1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydro-5-cyanoisobenzo Preparation of furan (formula II) oxalate
[0028] Add 19.1g (S)-(-)-4-[4-(dimethylamino)-1-(4-fluorophenyl)-1-hydroxy Butyl]-3-(hydroxymethyl)-benzonitrile, 17.4gK 2 CO 3 , add 200mL toluene, 80mL water, start stirring, and cool the system to 0°C with an ice-salt bath; add a solution of 8.4g methanesulfonyl chloride and 70mL toluene dropwise to the reaction solution through a constant pressure funnel, and control the temperature in the reaction bottle to 0°C, 2 The dropwise addition was completed within 1 hour and the reaction was controlled to be complete by TLC (thin layer chromatography). After the reaction was completed, the filtrate was filtered, and the filtrate was separated. The water layer was extracted once with 100 mL of toluene. The toluene layers were combined and washed twice with 100 mL of water. Distill the toluene to dryness under reduced pressure, add 100m...
Embodiment 2
[0029] Example 2 (S)-(+)-1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydro-5-cyanoisobenzo Preparation of furan (formula II) oxalate
[0030] Add 28.8g (S)-(-)-4-[4-(dimethylamino)-1-(4-fluorophenyl)-1-hydroxy Butyl]-3-(hydroxymethyl)-benzonitrile, 20.8gNa 2 CO 3 , add 200mL toluene, 80mL water, start stirring, and cool the system to 0°C with an ice-salt bath; add a solution of 17g p-toluenesulfonyl chloride and 150mL toluene dropwise to the reaction solution through a constant pressure funnel, and control the temperature in the reaction bottle to 0°C, 2 The dropwise addition was completed within 1 hour and the reaction was controlled to be complete by TLC (thin layer chromatography). After filtering, the filtrate was separated into layers, and the water layer was extracted once with 100 mL of toluene. The toluene layers were combined and washed twice with 100 mL of water. Distill toluene to dryness under reduced pressure, add 110mL of absolute ethanol and 16g of ...
Embodiment 3
[0031] Example 3 (S)-(+)-1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydro-5-cyanoisobenzo Preparation of furan (formula II) oxalate
[0032]Add 19.5 g of (S)-(-)-4-[4-(dimethylamino)-1-(4-fluorophenyl)-1-hydroxy Butyl]-3-(hydroxymethyl)-benzonitrile, 3.36g NaOH, add 80mL toluene, 20mL water, start stirring, cool the system to 0°C with an ice-salt bath; mix 8.5g p-toluenesulfonyl chloride and 65mL toluene solution The reaction solution was added dropwise through a constant pressure funnel, and the temperature in the reaction bottle was controlled at 0°C. After 2 hours, the dropwise addition was completed and the reaction was controlled by TLC (thin layer chromatography). After the reaction was completed, the filtrate was filtered, and the filtrate was separated. The water layer was extracted once with 50 mL of toluene. The toluene layers were combined and washed twice with 100 mL of water. Distill toluene to dryness under reduced pressure, add 60mL of absolute ethan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com