Treatment for intimal hyperplasia and related conditions
An intimal hyperplasia, mammalian technology, applied in the direction of disease, skin disease, sensory disease, etc., can solve the problem of time and money consuming inconvenience, increased risk of complications or death
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0130] general method
[0131] peptide synthesis
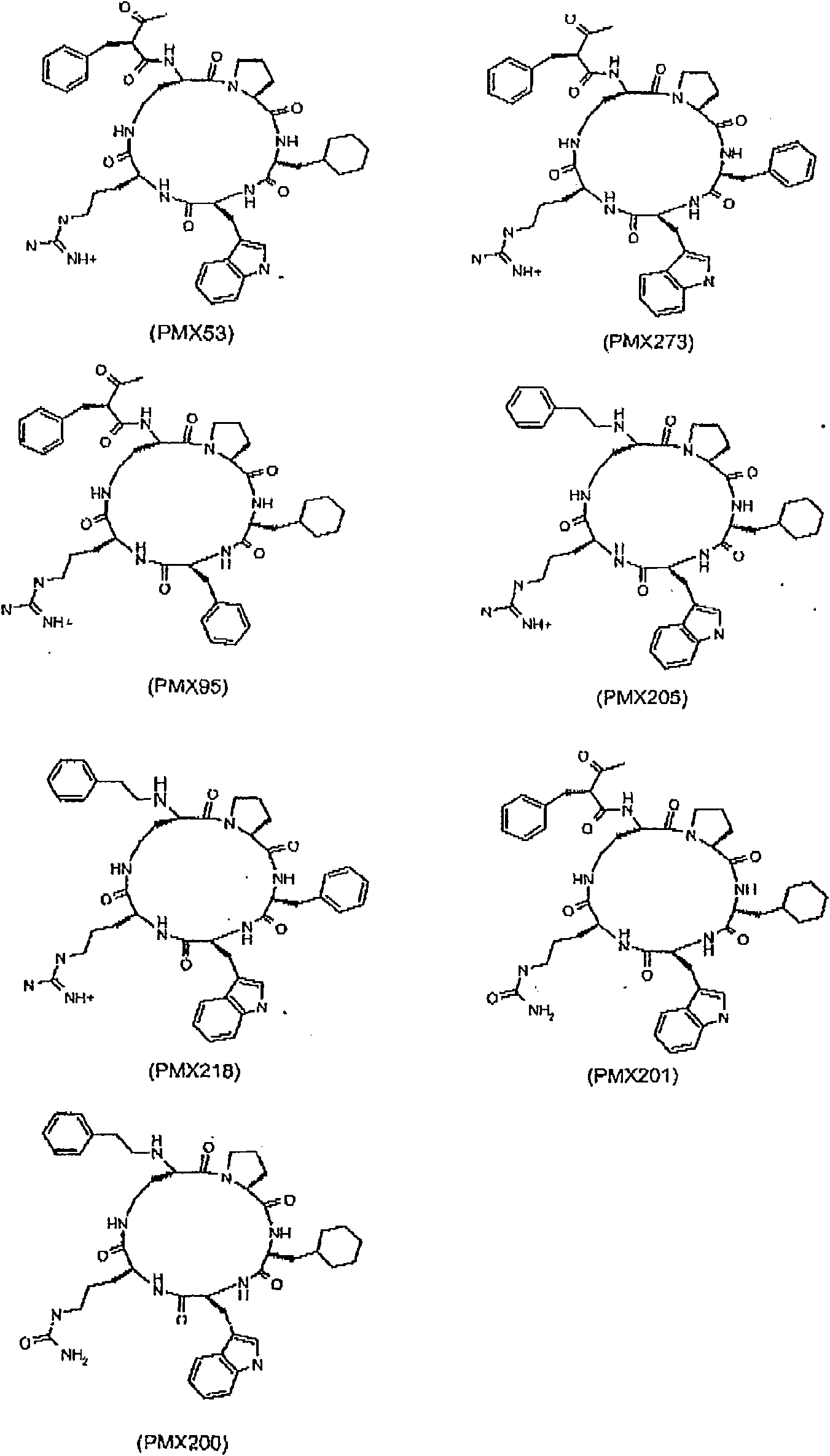
[0132] Cyclic peptide compounds of general formula I were prepared according to the methods detailed in our previous patent application numbers PCT / AU98 / 00490 (WO99 / 00406) and PCT / AU02 / 01427 (WO 2003 / 033528), both of which The entire content of is hereby incorporated by reference. Although using the reference compound AcF-[OPdChaWR](PMX53) (its corresponding linear peptide is Ac-Phe-Orn-Pro-dCha-Trp-Arg) and hydrocinnamate-[OP-(D-Cha)WR]( PMX205) (whose corresponding linear peptide is Hydrocinnamate-Orn-Pro-dCha-Trp-Arg) specifically illustrates the invention, but it should be clearly understood that the invention is not limited to these compounds.
[0133] Compounds 1-6, 17, 20, 28, 30, 31, 36 and 44 are disclosed in International Patent Application No. PCT / AU98 / 00490 (WO 99 / 00406) and for the first time in PCT / AU02 / 01427 (WO 2003 / 033528 Compounds 10-12, 14, 15, 25, 33, 35, 40, 45, 48, 52, 58, 60, 66 and 68-70 disclosed in...
Embodiment 2
[0164] C5 is present in remodeling vein grafts
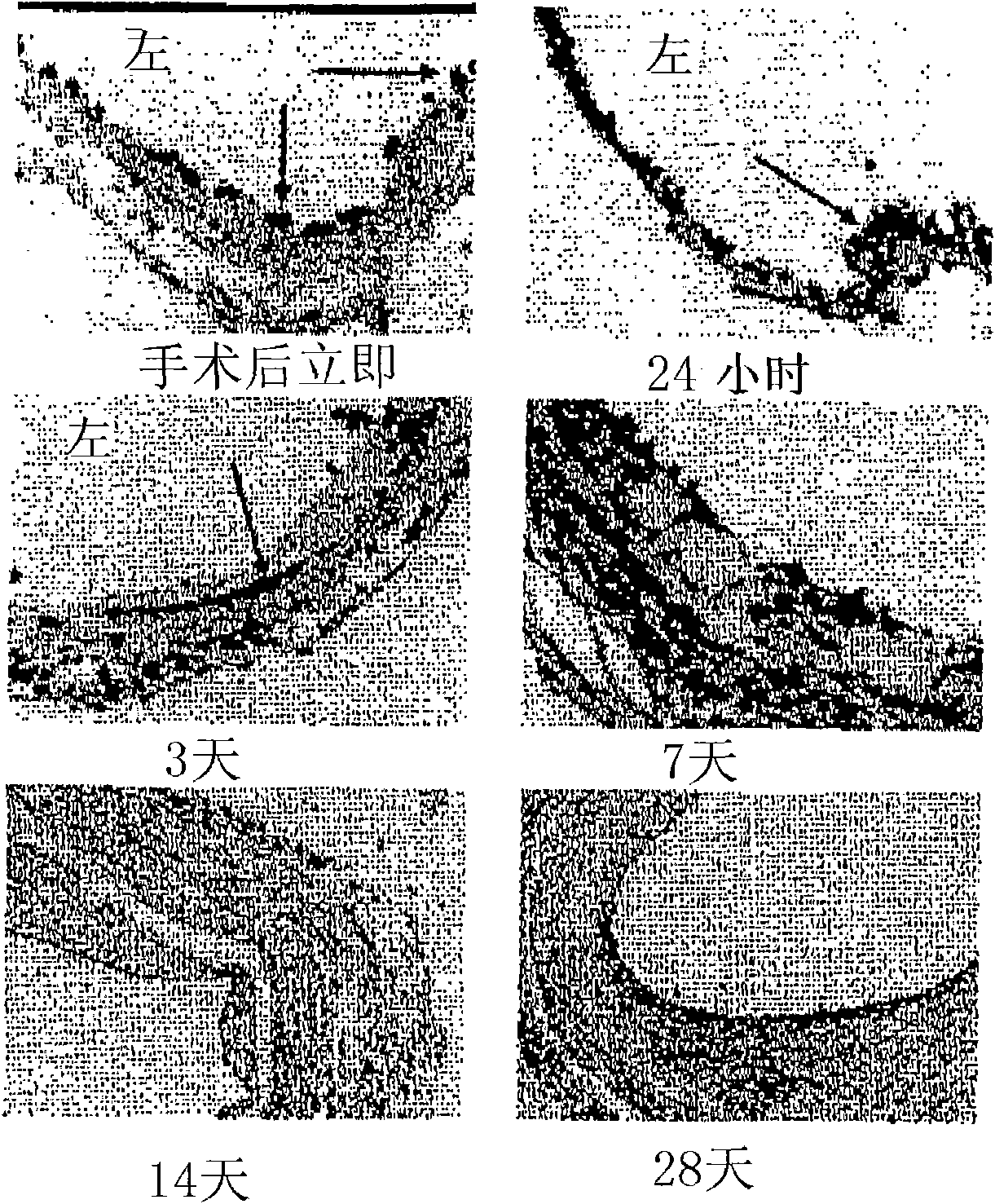
[0165] Detection of C5 in the intima by immunohistochemical analysis (n = 4 per time point) of vein grafts harvested at several time points after surgery (t = 6 hours, 1, 3, 7, 14 and 28 days) The presence of proliferative development.
[0166] In vein grafts harvested 6 hours and 1 day postoperatively, large amounts of C5 could be detected in adherent monocytes and adventitial fibroblasts. C5 was also detected in the regenerated endothelium 7 days after surgery. At this stage, staining appears to be most prominent and widespread, suggesting the presence of abundant C5 in the vessel wall. At later time points (postoperative days 14 and 28), C5 expression paralleled the development of intimal hyperplasia was observed in endothelial cells, adherent monocytes, adventitial fibroblasts, and foam cells. There are a small number of smooth muscle cells that show positive staining for C5, as in figure 2 shown.
Embodiment 3
[0168] Time-dependent expression of C5a receptor mRNA in remodeled vein grafts
[0169] To determine whether C5a receptors were present and produced in vein grafts, total RNA was isolated and detected by RT-PCR for the presence of C5aR mRNA. Vein grafts were harvested at several time points (t=24 hours, 3 days, 7 days and 28 days, n=4 for each time point), as well as normal vena cava as controls. Total cDNA in all samples was determined by the presence of the housekeeping gene β-actin.
[0170] Normal vena cava showed little expression of C5aR. The expression of C5a increased in a time-dependent manner in vein grafts. Peak expression was observed at 7 days postoperatively, after which expression decreased to levels observed in normal vena cava at 28 postoperative days; image 3 explained in.
[0171] These data confirm that C5a receptors are present in vein grafts and are upregulated early in the vein graft thickening process.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com