Application of traditional Chinese medicine composition in preparing antihypoxic
A technology of composition and traditional Chinese medicine, which is applied in the application field of traditional Chinese medicine composition in the preparation of anti-hypoxic drugs, and can solve problems such as the application of undisclosed traditional Chinese medicine composition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] a) The raw material drug formula is:
[0045] Ginseng 39.6g Leech 72.6g Wood Beetle 46.2g Frankincense (made) 13.2g
[0046] Paeoniae Rubra 33g Dalbergia 13.2g Sandalwood 13.2g Scorpio 19.8g
[0047] Cicada slough 46.2g centipede 6.6g borneol 33g Suanzaoren (stir-fried) 33g;
[0048] b) Crushing process of medicinal materials:
[0049] The scorpion, leech, centipede, ground beetle, and cicada slough five kinds of insect medicines have been cleaned, washed and processed, and the frankincense prepared after cleaning is prepared according to the prescription, and crushed by a pulverizer, and the fineness of the powder reaches more than 80 mesh; The powdered medicine is ultrafinely pulverized by various ultrafine pulverization techniques, so that the average particle size of the medicine powder is less than 30-40 μm; the medicinal materials to be pulverized are washed, dried and sterilized, and the ingredients are prepared;
[0050] c) Extraction concentration and drying...
experiment example 1
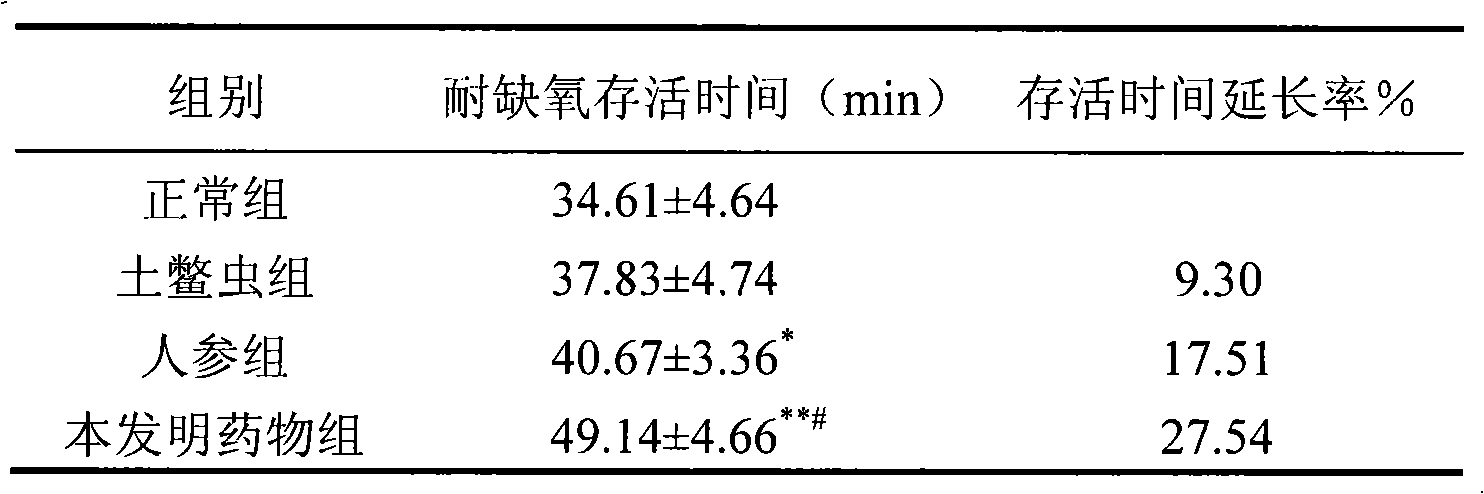
[0056] Experimental Example 1 is a classic test of the main pharmacodynamics of anti-hypoxic drugs, which can confirm whether the drug has an anti-hypoxic effect.
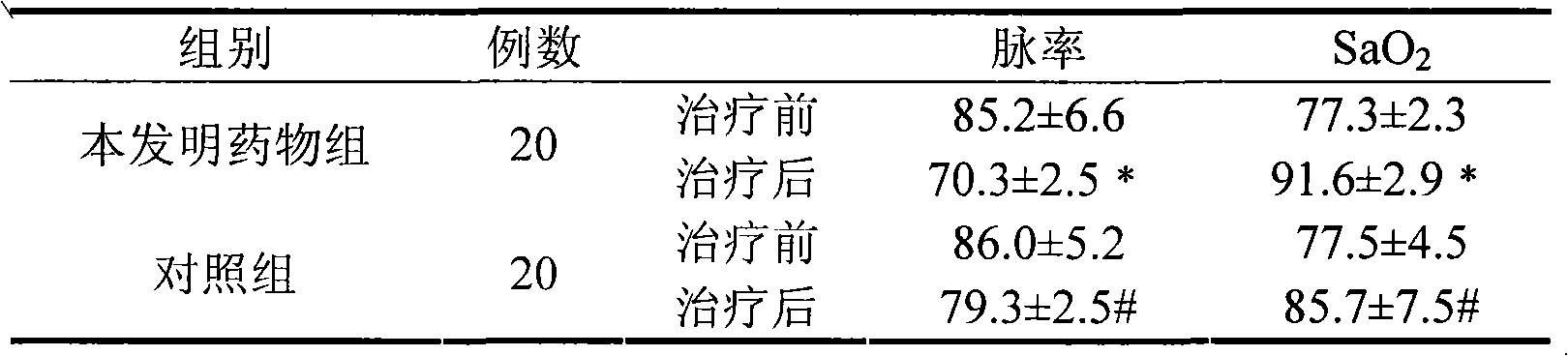
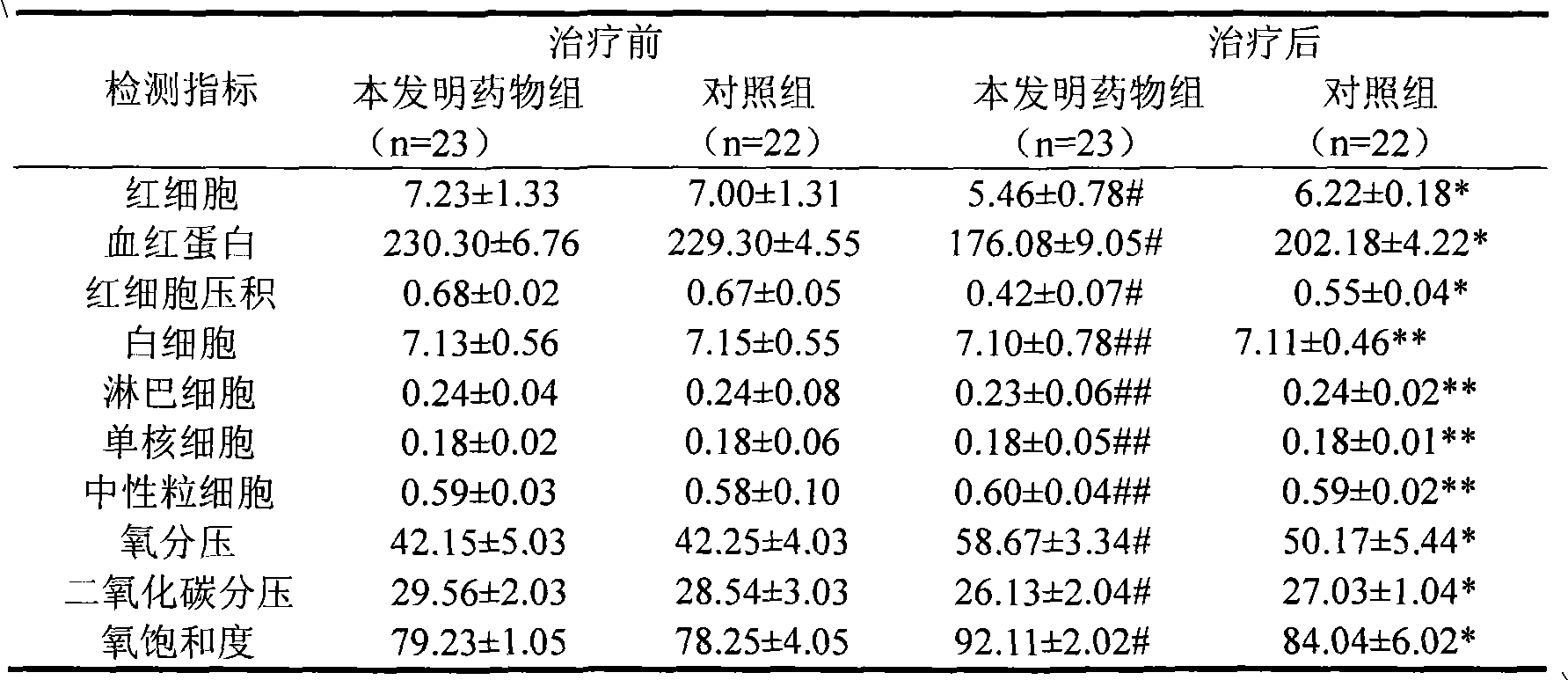
[0057] Experimental example 2 and experimental example 3 are to carry out clinical test with the medicine of the present invention, and these two tests prove that the medicine has therapeutic and preventive activity for various diseases caused by altitude sickness and hypoxia operation.
[0058] Experimental example 1:
[0059] 1 Materials and methods
[0060] 1.1 Experimental animals: Healthy KM mice, half male and half female, weighing 26-28 g, were purchased from the Experimental Animal Center of Hebei Province, license number: SCXK (Ji) 2003-1-003. Animal identification was marked with 5% picric acid, and mice were kept in cages, 5 mice / cage. Rats were fed full-price pellets and adequate drinking water was maintained. Adaptive feeding for three days.
[0061] 1.2 Main drug: the drug of the present invention...
experiment example 2
[0075] 1 Materials and methods
[0076] 1.1 General information All cases are all selected from the inpatients of Golmud Railway Hospital in Qinghai Province, a total of 85 cases, randomly divided into two groups of drug group of the present invention and control group. The medicine group of the present invention includes 43 cases, including 40 males and 3 females, with an average age of 40 years, 6 cases of acute altitude sickness, 6 cases of high-altitude cerebral edema, 8 cases of high-altitude pulmonary edema, 3 cases of high-altitude heart disease, and 2 cases of high-altitude hypertension , 1 case of high altitude hypotension, 8 cases of high altitude polycythemia, 9 cases of mixed type of chronic high altitude sickness; 42 cases of control group, including 39 males and 3 females, with an average age of 39 years, 4 cases of acute high altitude sickness, high altitude cerebral edema 6 cases, 10 cases of high-altitude pulmonary edema, 2 cases of high-altitude heart disease...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com