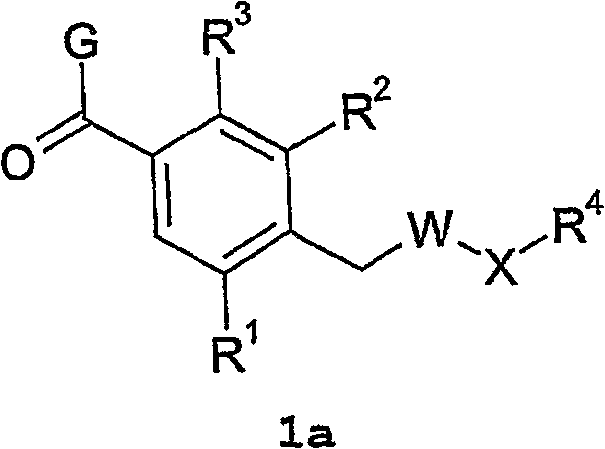
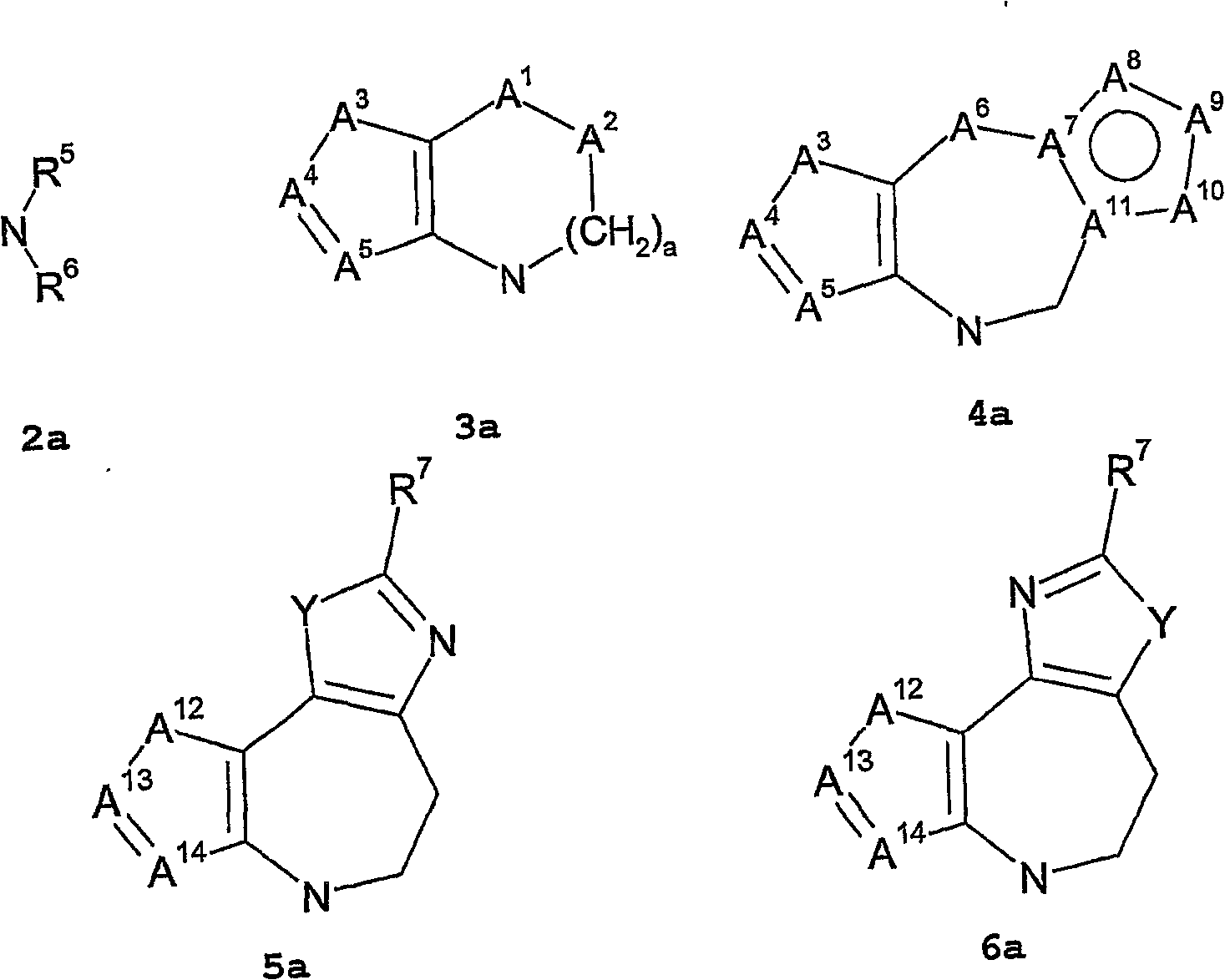
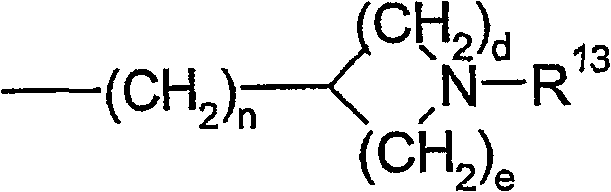
Vasopressin v1a antagonists
A c-r12, alkyl technology, applied in the field of vasopressin V1a antagonists, can solve problems such as no reported activity of V antagonists
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0125] Use the following abbreviations:
[0126] AIBN 2,2′-Azobisisobutyronitrile
[0127] Boc tert-butyl carboxylate or tert-butoxycarbonylamino
[0128] Bu butyl-alkyl residues can be further expressed as n (positive, that is, straight chain), i (iso)
[0129] And t (uncle)
[0130] DIEA N, N-Diisopropylethylamine
[0131] DMAP 4-(dimethylamino)pyridine
[0132] DMF Dimethylformamide
[0133] Et ethyl
[0134] EtOAc ethyl acetate
[0135] HOBt 1-Hydroxybenzotriazole
[0136] HPLC high performance liquid chromatography
[0137] H hour(s)
[0138] Me methyl
[0139] Min minute(s)
[0140] MS mass spectrometry
[0141] NMR nuclear magnetic resonance spectroscopy-unless otherwise indicated, NMR spectra are recorded in CDCl3,
[0142] The frequency is 270MHz
[0143] OVA Ornithine Pressor Oxytocin Analog
[0144] Petroleum ether with a boiling point of 60-80℃
[0145] Ph phenyl
[0146] Pn pentyl
[0147] Pr propyl
[0148] THF Tetrahydrofuran
[0149] Tos toluene-4-sulfon...
Embodiment E1
[0154] 6-Chloro-3-methyl-3,4,9,10-tetrahydro-2,3,4,9-tetraaza-benzo[f]azulene
Embodiment E11
[0156] 5-(4-Chloro-2-nitro-phenylamino)-1-methyl-1H-pyrazole-4-carboxylic acid ethyl ester.
[0157] Add sodium hydride (60% dispersion in oil, 7.28g, 180mmol) to ethyl 5-amino-1-methylpyrazole-4-carboxylate (21.8g, 148mmol) in portions at 0°C. Solution in anhydrous THF. The mixture was allowed to warm to room temperature and stirred for 1 h. A solution of 4-chloro-2-fluoronitrobenzene (22.6 g, 129 mmol) in dry THF (50 ml) was added dropwise. The obtained dark purple solution was stirred at room temperature for 18h and then poured into ice-cold 1N hydrochloric acid. The obtained mixture was extracted with dichloromethane (200 ml×2), and the combined organic extracts were washed with brine and concentrated in vacuo. The residue was purified by flash silica gel chromatography (eluent; 60% petroleum ether: 40% EtOAc) to obtain the title compound (27.5 g, 62%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com