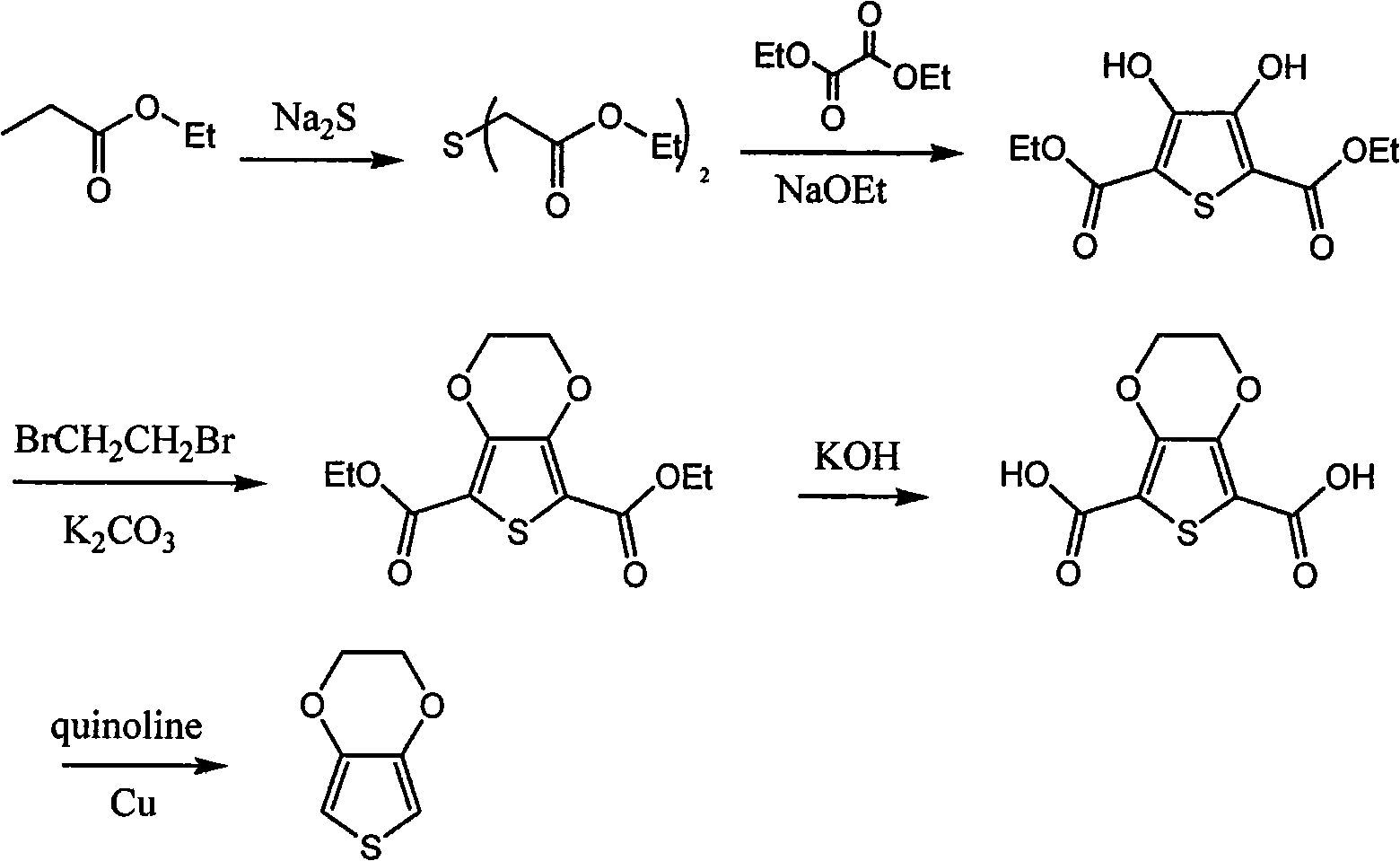
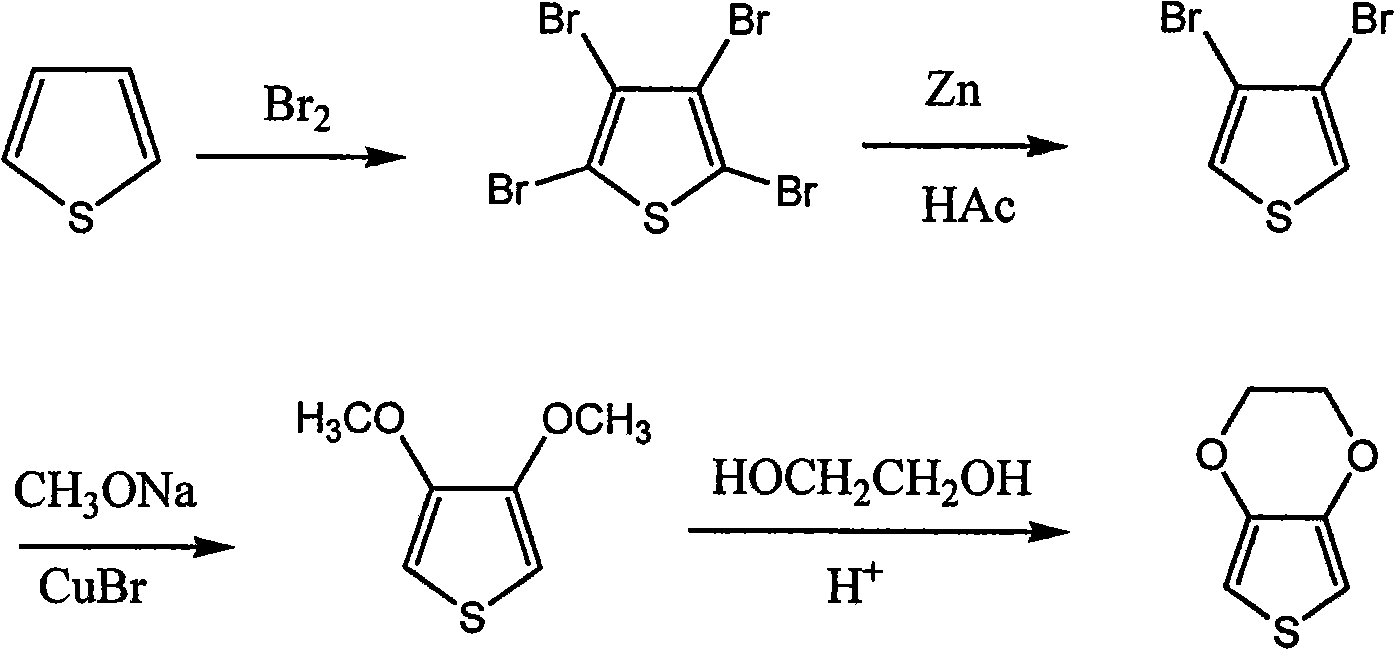
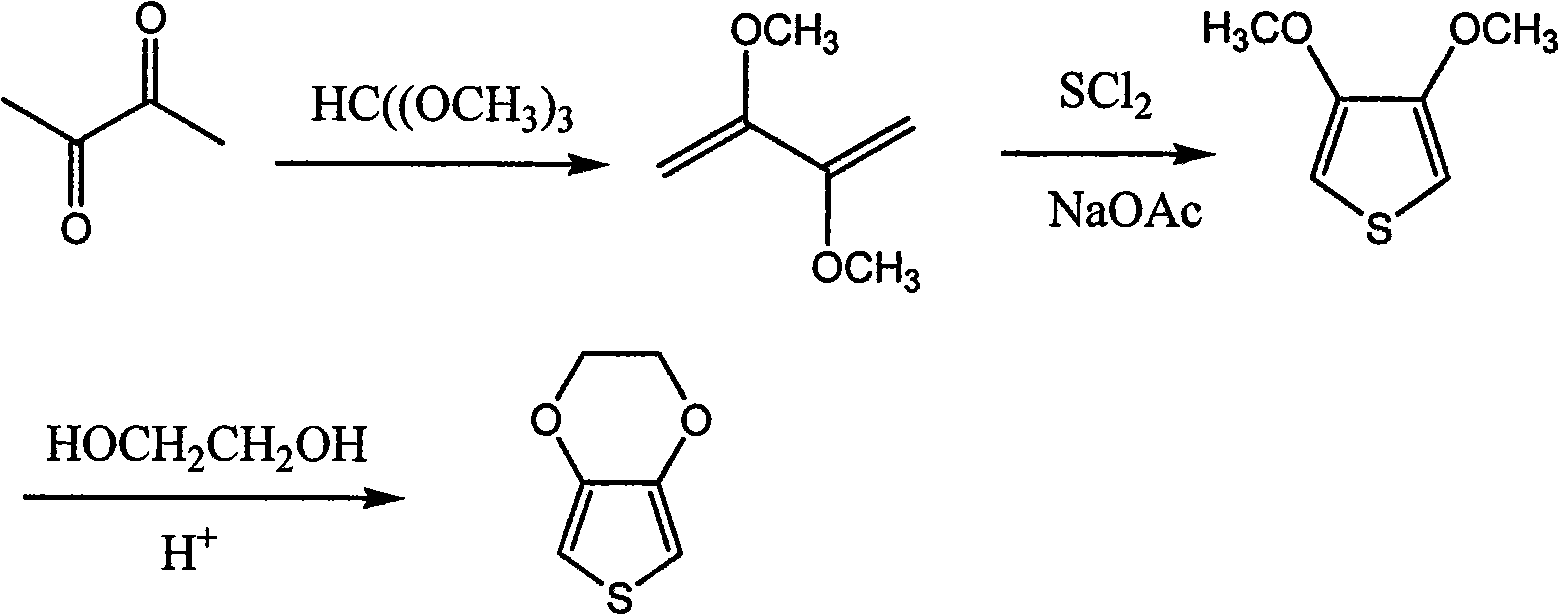
Synthetic process of 3, 4-ethylenedjoxythiophene
A technology of ethylenedioxythiophene and synthesis process, applied in 3 fields, can solve the problems of unstable product, influence product yield and quality, long reaction route, etc., and achieve the effect of simplifying synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 1. Preparation of disodium ethylene glycol
[0024] 31 grams (0.5 moles) of ethylene glycol and 40 grams (1.0 moles) of sodium hydroxide were dissolved in 500 milliliters of toluene, and refluxed for 1.5 hours. After the reaction, the toluene and water were distilled off, and the toluene and water were further removed by vacuuming. , and then dried at 130°C for 0.5 hours to obtain disodium ethylene glycol.
[0025] 2. Preparation of 3,4-ethylenedioxythiophene
[0026] Cool 53 grams of disodium ethylene glycol obtained in Step 1 to 50° C., and add 500 milliliters of N,N-dimethylformamide, 109 grams (0.45 moles) of 3,4-dibromothiophene, and bromine 13 g (0.09 mol) of cuprous chloride was heated up to 110° C., and reacted at this temperature for 5 hours. After the reaction, the reaction solution was poured into 2 liters of water, and the product was extracted with toluene; after the extraction, the toluene solution was distilled to dryness to obtain 50 g of 3,4-ethylened...
Embodiment 2
[0028] 1. Preparation of disodium ethylene glycol
[0029] Using ethylene glycol and sodium as raw materials, it is prepared according to the existing technology.
[0030] 2. Preparation of 3,4-ethylenedioxythiophene
[0031] Put 53 grams of disodium ethylene glycol obtained in step 1 into a reaction vessel, add 500 milliliters of N-methylpyrrolidone, 109 grams (0.45 moles) of 3,4-dibromothiophene, and 36 grams (0.45 moles) of copper oxide and potassium iodide 0.82 g (0.005 mol), the temperature was raised to 50° C., and the reaction was carried out at this temperature for 24 hours. After the reaction, the reaction solution was poured into 2 liters of water, and the product was extracted with toluene; after the extraction, the toluene solution was distilled to obtain 55 grams of the product, with a yield of 86%.
Embodiment 3
[0033] 1. Preparation of disodium ethylene glycol
[0034] The preparation method is the same as in Example 1.
[0035] 2. Preparation of 3,4-ethylenedioxythiophene
[0036] Cool 53 grams of disodium ethylene glycol obtained in step 1 to 50°C, and add 500 milliliters of N-methylpyrrolidone, 109 grams (0.45 moles) of 3,4-dibromothiophene, and 17.14 cuprous iodide to the reaction vessel. g (0.09 moles), the temperature was raised to 150° C., and the reaction was carried out at this temperature for 5 hours. After the reaction, the reaction solution was poured into 2 liters of water, and the product was extracted with toluene; after the extraction, the toluene solution was distilled to obtain 51 grams of the product, with a yield of 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com