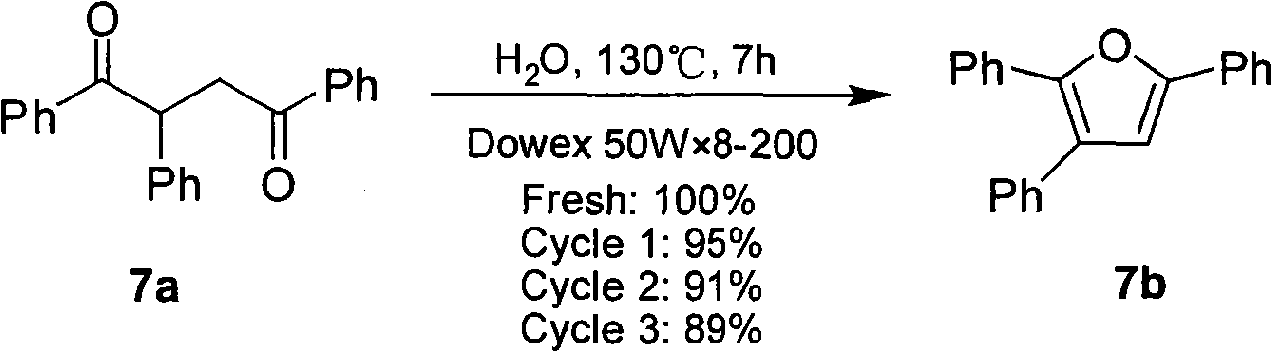Green synthesis method of furane derivative
A multi-substituted furan, green synthesis technology, applied in the field of green synthesis of multi-substituted furan, can solve problems such as not meeting the development requirements, and achieve the effects of easy recycling, lower production costs, and lower environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0011] In order to make the object, technical solution and advantages of the present invention clearer, preferred embodiments of the present invention are described in detail below.
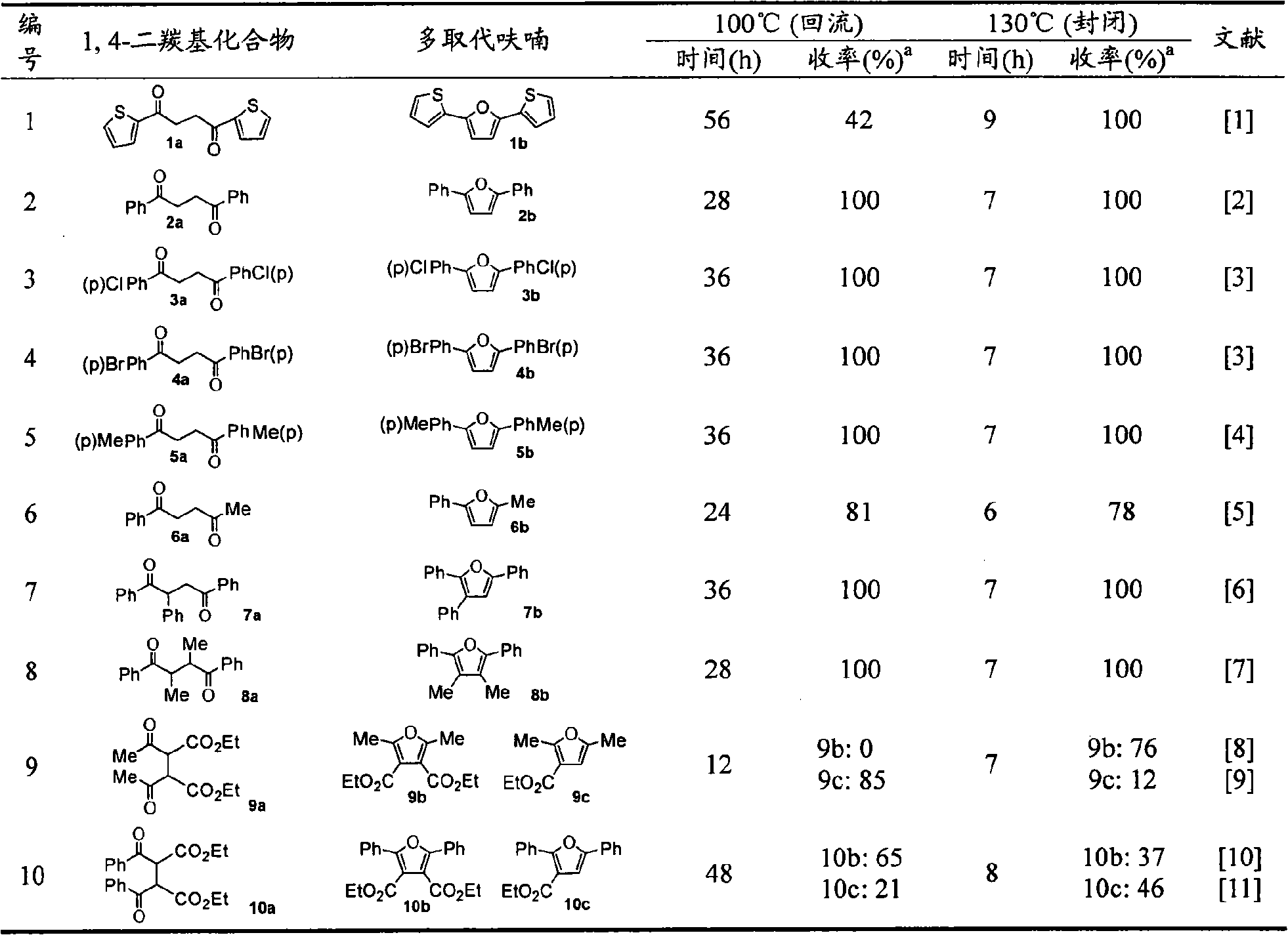
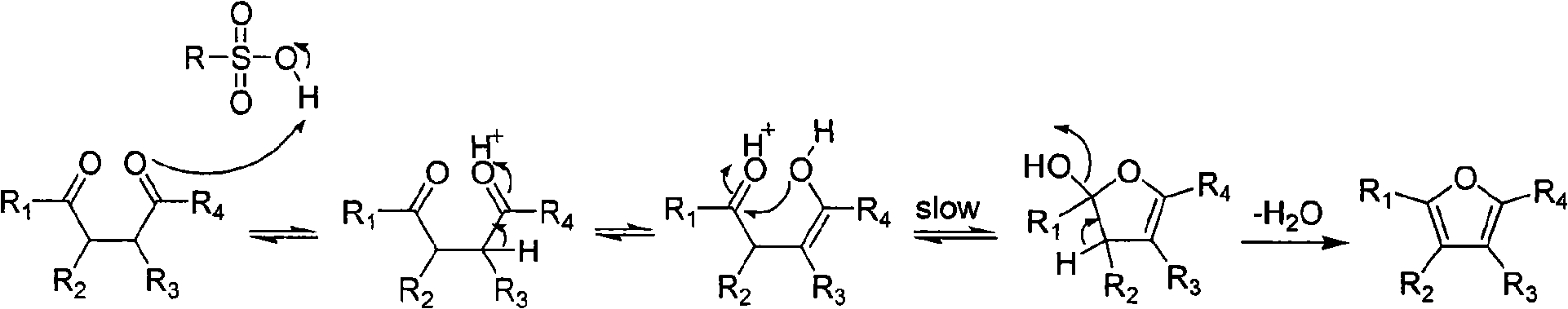
[0012] In a preferred embodiment, the raw material 1,4-dicarbonyl compounds 1a, 3a~5a and 8a~10a are prepared according to the method in related literature: 1a (Nakazaki J et al.J.Mater.Chem., 2003, 13, 1011.) , 3a, 4a and 8a (Ceylan M et al. Synthesis, 2004, 1750.), 5a (Joczyk A et al. Tetrahedron, 1990, 46, 1025.), 9a (Gatezowski M et al. J. Org. Chem. , 2006,71,5942.), 10a (Wu AX et al.Synth.Commun., 1997,27,331.); 2a, 6a and 7a were purchased from Alfa Aesar Company; Reagents were all commercially available and without further Purification; in the reaction, TLC (GF254 silica gel plate) was used to monitor the reaction process, and the obtained target product was measured by X-4 type micro melting point instrument (digital display) (the temperature was not corrected), Bruker AV-300 type nuclea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com