Reaction system and method for generating flavanone by cyclization of 2'-hydroxy chalcone
A technology of hydroxychalcone and reaction system, applied in the direction of organic chemistry and the like, can solve the problems of poor selectivity, high cost and high yield of flavanones, and achieve the effects of easy popularization, simple operation and high-efficiency synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: 2'-hydroxychalcone heating cyclization
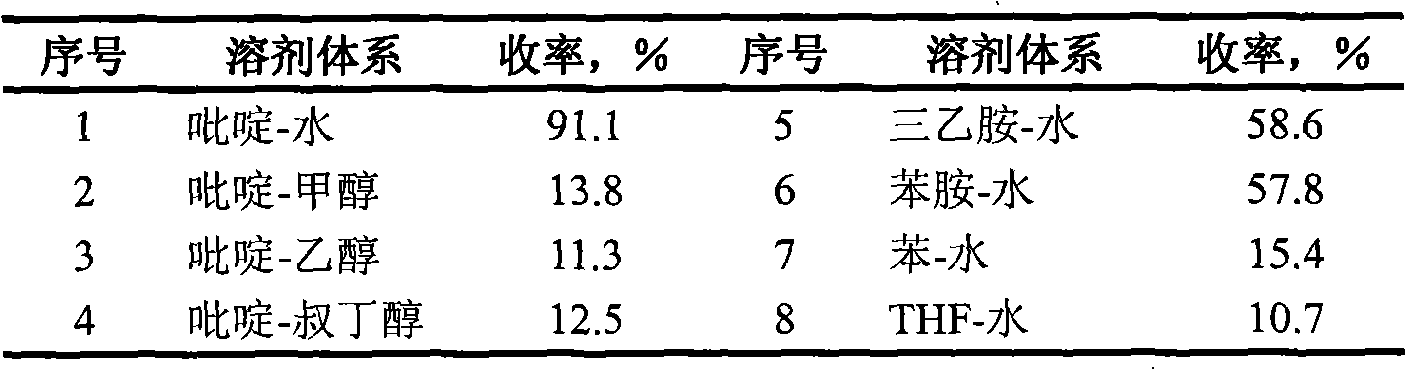
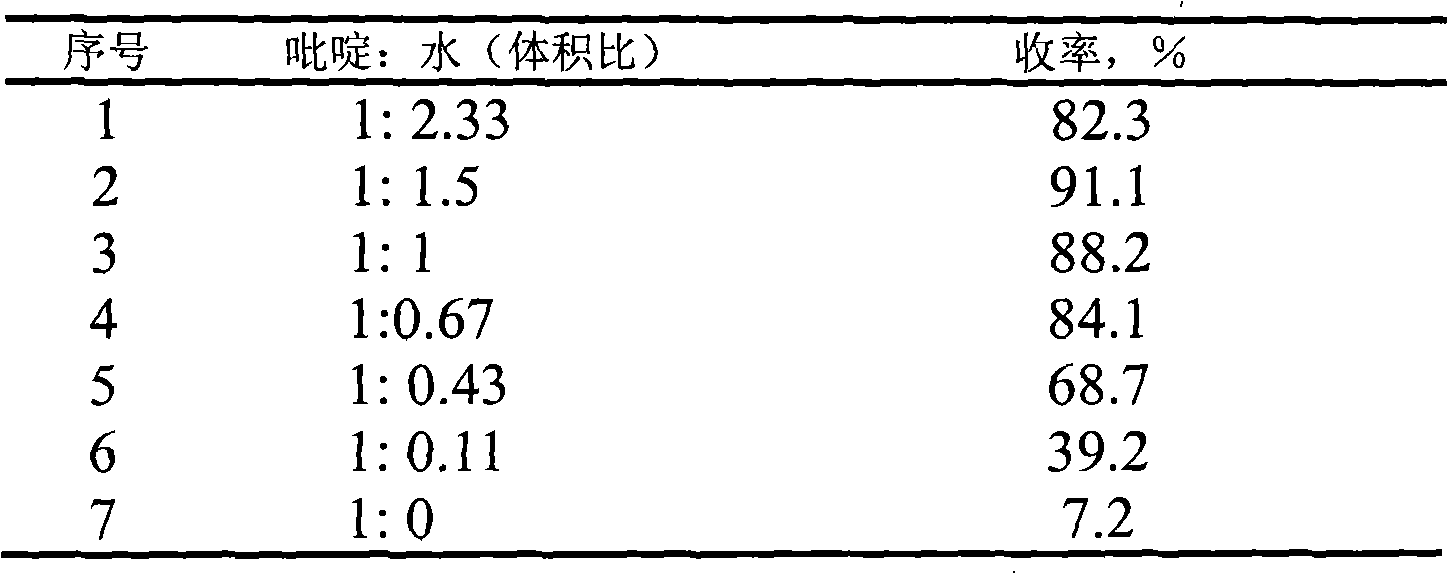
[0030] Weigh 1mmol of 2'-hydroxychalcone, dissolve it with 40ml of pyridine, add it to a 250ml three-neck round bottom flask, add 60ml of distilled water, stir mechanically, condense and reflux, control the temperature at 90°C, and react for 1h; concentrate under reduced pressure; Column chromatography separation (eluent is benzene: ethyl acetate=30: 1), distillation under reduced pressure; Concentrate is constant volume with methanol, adopts ultraviolet-visible spectrophotometer to measure absorbance at 251nm place of characteristic peak of flavanone, The flavanone content was calculated, and the yield was 91.1%; and the melting point, IR, H-NMR, and HRMS analyzes were carried out.
[0031] Other solvent systems (volume ratio is 1: 1.5), are shown in Table 1 to the action effect of heating cyclization of 2'-hydroxychalcone under the above-mentioned same conditions.
[0032] Table 1 Effect of different solvent systems...
Embodiment 2
[0039] Example 2: cyclization of 2'-hydroxychalcone with visible light
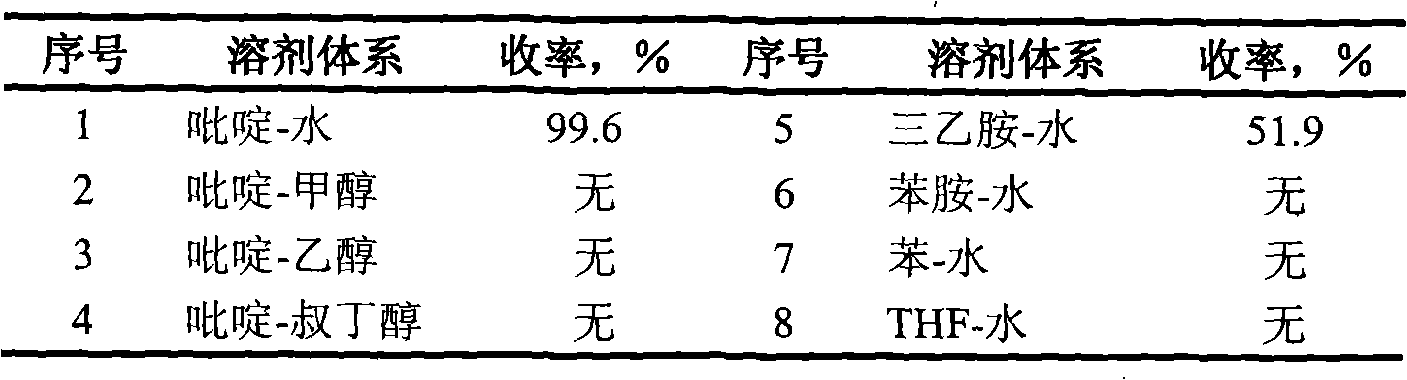
[0040] Visible light photocyclization of 2'-hydroxychalcone was studied in pyridine-water system. Weigh 1mmol of 2'-hydroxychalcone, dissolve it with 40ml of pyridine, pour it into a quartz glass photoreactor, add 60ml of distilled water, fix the quartz glass photoreactor on a shaker, irradiate with a 500W xenon lamp, and react 4h, the reaction mixture was concentrated under reduced pressure, separated by column chromatography (eluent: benzene:ethyl acetate=30:1), and distilled under reduced pressure; The absorbance was measured at a fixed wavelength at 251nm, the characteristic peak of alkanone, and the content of flavanone was calculated. The yield was 99.6%; and analyzed by melting point, IR, H-NMR, HRMS and so on.
[0041] The effects of different reaction systems on the visible light photocyclization of 2'-hydroxychalcone to generate flavanones under the same conditions above are shown in Table 3. ...
Embodiment 3
[0045] Example 3: Photocyclization of 2'-hydroxychalcone under natural light
[0046] The natural light photocyclization reaction of 2'-hydroxychalcone was studied in pyridine-water system. Weigh 1mmol of 2'-hydroxychalcone, dissolve it with 40ml of pyridine, add it to a 250ml three-necked round-bottomed flask, then add 60ml of distilled water, stir mechanically, and react for 4 hours under natural light in the room, concentrate the reaction mixture under reduced pressure, and perform column chromatography Separation (eluent is benzene: ethyl acetate = 30: 1), distillation under reduced pressure; Concentrate is distilled to volume with methanol, adopts ultraviolet-visible spectrophotometer to measure absorbance at fixed wavelength at 251nm place of flavanone characteristic peak, calculates Flavanone content, the yield is 89.7%; and analyzed by melting point, IR, H-NMR, HRMS and so on.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com