Method for preparing ursodeoxycholic acid through cyclic enzyme catalysis
A technology for ursodeoxycholic acid and catalytic preparation is applied in the field of catalyzed preparation of ursodeoxycholic acid by circulating enzymes, which can solve the problems of high cost of sewage treatment, non-compliance with environmental protection policies, unfavorable large-scale stable production, etc., and achieves easy recovery and treatment. , The effect of reducing subsequent processing procedures and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
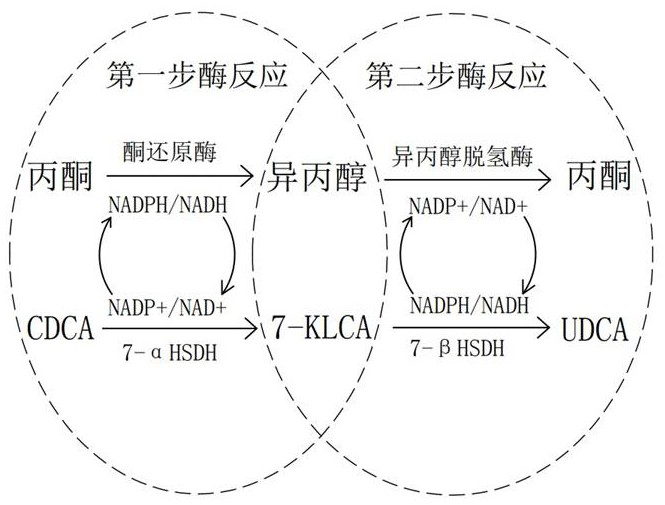
[0026] Example 1: Reaction system 500ml, first add 120ml of acetone and 250ml of pure water into a 1L three-hole reaction bottle and stir to mix. The reaction bottle is placed in a 25°C constant temperature water bath, then add 50.16g of CDCA and continue to stir for half an hour, let Dissolve it fully into the mixed solution, then add 0.02g NADP+, adjust the pH to 6.0-6.2, then add 5.42g ketoreductase and 15.28g 7-αHSDH, make up the volume with pure water, react at 300rpm for 12-15h, control during the reaction The temperature and pH are within the range of process requirements, and the content of CDCA and 7-KLCA is detected by liquid phase. When the substrate reaction exceeds 98%, the reaction is terminated. The reaction liquid is passed through a 60-mesh filter to recover the immobilized enzyme, and the rest of the system is waiting to enter the second step enzyme reaction.
Embodiment 2
[0027] Example 2: The reaction system is about 550ml. First, add the first step enzyme reaction solution prepared in Example 1 into a 1L three-hole reaction bottle, place the reaction bottle in a 30°C constant temperature water bath, turn on the stirring at 300rpm and stir for half an hour. Add 50ml of isopropanol to the reaction solution, adjust the pH to 7.0-7.2, then add 15.31g of isopropanol dehydrogenase and 25.22g of 7-βHSDH, react at 300rpm, control the temperature and pH during the reaction within the range of process requirements, and use The content of 7-KLCA and UDCA was detected by liquid phase. When the substrate reaction exceeded 98%, the reaction was terminated. The reaction liquid passed through a 60-mesh filter to recover the immobilized enzyme. ml, 82°C vacuum recovery isopropanol, to obtain isopropanol 45.5ml.
[0028] After the organic phase was removed from the reaction solution, it was extracted three times with 380ml ethyl acetate, the organic phases wer...
Embodiment 3
[0029] Example 3: The reaction system is 1000L, first add 240L acetone and 500L pure water into a 1.5T stainless steel reaction tank, turn on the stirring at 200rpm, and control the temperature of the jacket at 30°C, then add 100.09kg CDCA and continue stirring for half an hour to allow it to fully dissolve To the mixed solution, then add 0.05kg NADP+, adjust the pH to 7.0-7.2, then add 15.35kg ketoreductase and 50.07kg 7-αHSDH, make up the volume to 1000L with pure water, and react at constant temperature. During the reaction, control the temperature and pH at Within the range of process requirements, the content of CDCA and 7-KLCA is detected by liquid phase. When the substrate reaction exceeds 98%, the reaction is terminated. The reaction solution passes through a 60-mesh filter to recover the immobilized enzyme, and the rest of the system waits for the second step of enzyme reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com