Alpha-lactalbumin composition
A technology of whey protein and composition, which is applied in the direction of drug combination, albumin peptide, peptide/protein composition, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
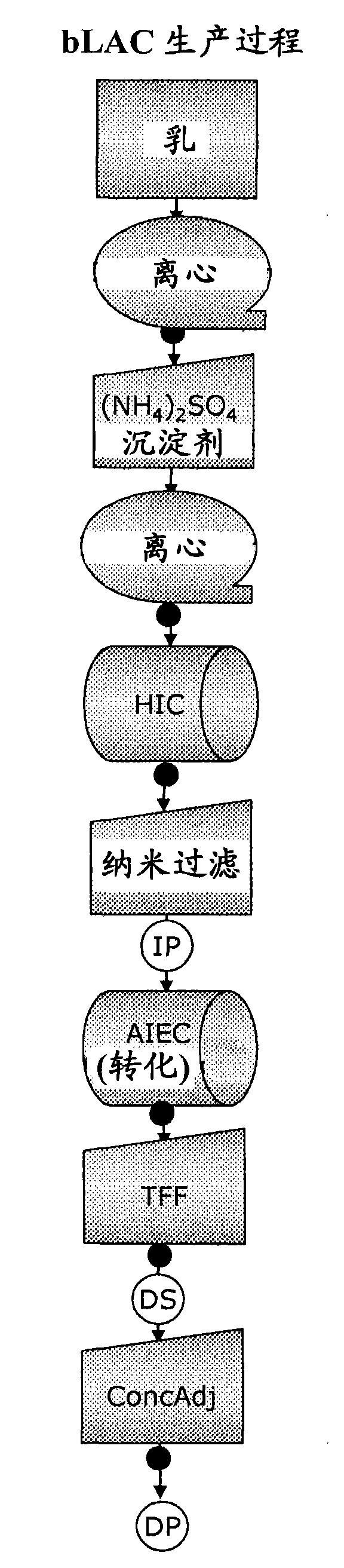
[0426]Purification and conversion of bovine α-lactalbumin into bLAC
[0427] Whole milk (2 L) was skimmed by centrifugation at 3500 xg for 30 minutes. Skim milk was subjected to ammonium sulfate precipitation overnight (264 g / L = 40-45% saturation), after which the precipitate was centrifuged at 3500 xg for 30 minutes. The ammonium sulfate precipitated supernatant was harvested and filtered first through a paper filter to remove any remaining precipitate or fat, then through a Millipore Optiscale filter with Polysep II media 1.0 / 0.5 μm.
[0428] The ammonium sulfate precipitated supernatant was made suitable for HIC chromatography by adding 32.55 mL EDTA (0.25M) + 24.97 mL Tris-EDTA (Tris 50 mM, EDTA 1 mM, pH 7.5) + 67.45 mL per 100 mL ammonium sulfate precipitated supernatant. Conditioned medium was adjusted to pH 7.5.
[0429] By filling 300mL phenyl sepharose 6FF High Sub (GE Healthcare) with a bed height of 15cm and an area of 19.6cm 2 Bovine α-lactalbumin was purifie...
Embodiment 2
[0449] Cytotoxicity of monomeric α-lactalbumin compositions
[0450] As outlined below and in the Figure 4 As shown in, by adopting ViaLight from Cambrex PLUS cell proliferation and cytotoxicity kit (Cell Proliferation and Cytotoxicity BioAssay Kit), test the cytotoxic activity of α-lactalbumin composition by viability test. The potency of LAC preparations is derived from their killing of the murine lymphocytic leukemia cell line L1210 (ATCC Competence Assay Cat. No. CCL-219).
[0451] For each LAC formulation tested, an appropriate serial dilution was prepared in 0.9% NaCl solution. Mix 20 μL of each dilution (in triplicate) on a 96-well white-walled cell culture plate with 50 μL of cell suspension containing 100,000 or 200,000 PBS-washed L1210 cells in RPMI 1640 medium without serum and HEPES . at 37°C and 5% CO 2 After 1 hour of incubation at 0°C, 5 μL of fetal calf serum was added to each well to inactivate all extracellular LACs. at 37°C and 5% CO 2 After an addi...
Embodiment 3
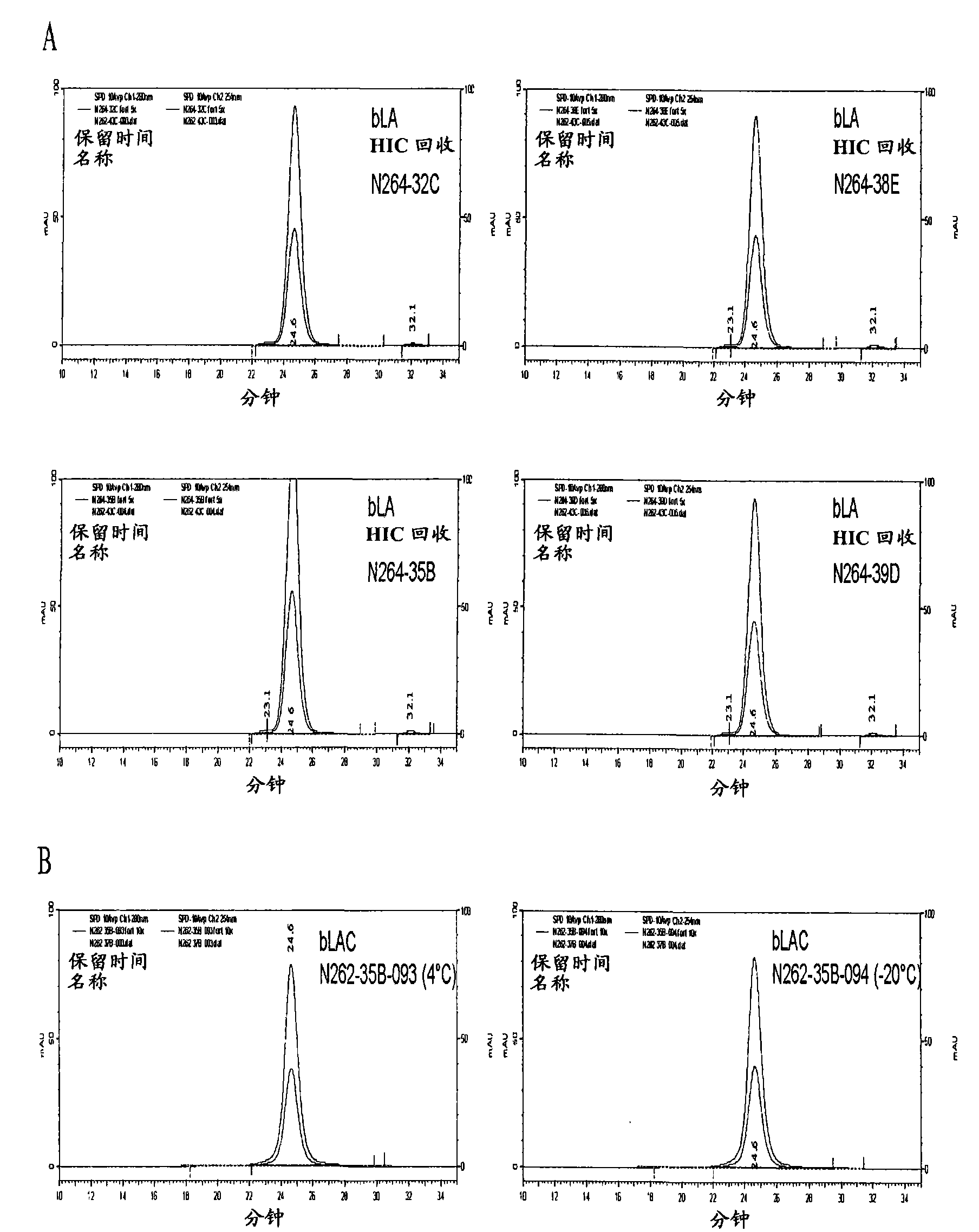
[0460] HIC loading as a determinant of monomeric / multimeric bLAC ratio in α-lactalbumin complexes
[0461] The monomer / polymer bLAC ratio can be controlled by the monomer / polymer composition of bLA purified by hydrophobic interaction chromatography as described in Example 1.
[0462] 2mg / mL gel (32mg / cm 2 ) loading resulted in bLA containing both dimeric and monomeric forms ( Figure 8 ), while 6mg / mL resin (90mg / cm 2 ) loading result is only containing monomer bLA (embodiment 1, image 3 ). Transformation of bLA was performed as described in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com