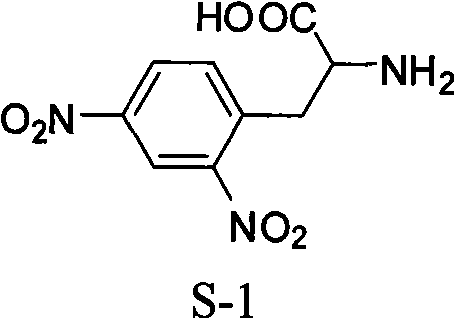
Method for synthesizing L-2,4-dinitrophenylalanine
A technology of dinitrophenylalanine and nitrophenylalanine, which is applied in the field of L-2, can solve problems such as deamination or side chain breakage, and achieve the effects of less side reactions, simple process and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Embodiment 1, the synthesis of urea nitrate (belonging to known technology)
[0016] Add 120g of urea and 80mL of water into a 500mL three-necked flask, heat to 50-60°C, stir to completely dissolve the urea in the water, and add 210g of nitric acid with a concentration of 65% (wt) drop by drop. After reacting at ℃ for 1 hour, the flask was placed in an ice bath, and solids precipitated out. After filtering and drying, urea nitrate was obtained, m.p.151.7~152.6℃.
Embodiment 2
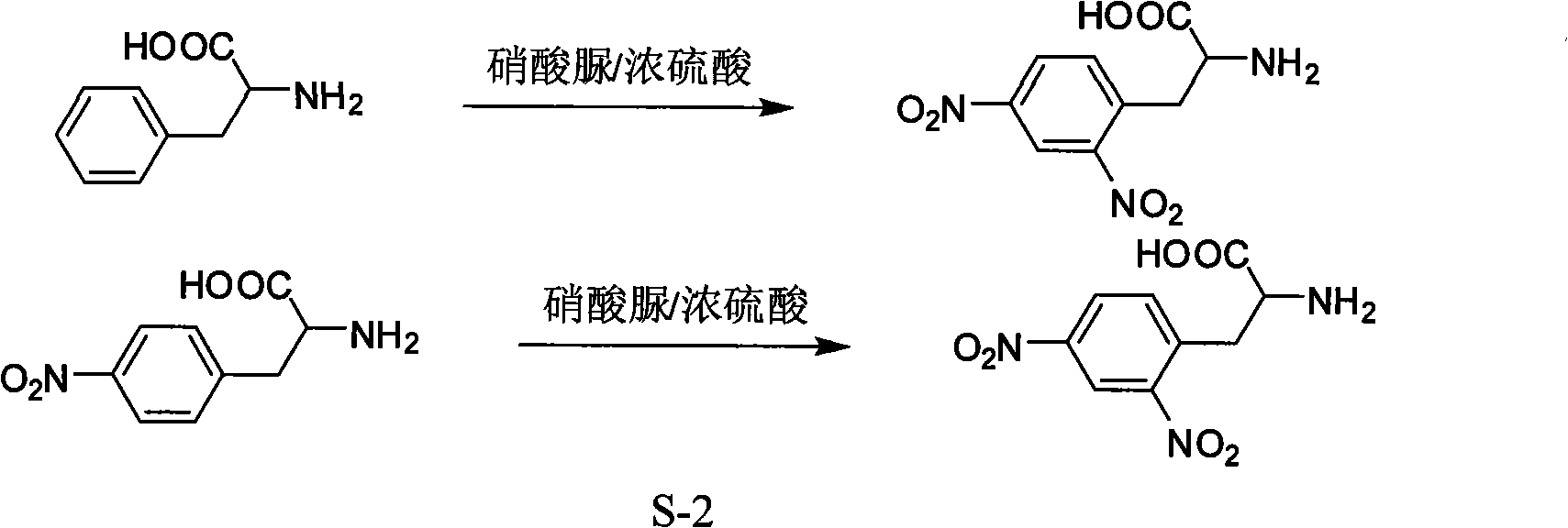
[0017] The synthesis of embodiment 2, L-4-nitrophenylalanine (belongs to known technology)
[0018] Add 120mL of nitric acid with a concentration of 65% (wt) in a 500mL three-necked flask, then add 120mL of concentrated sulfuric acid drop by drop under ice bath, add 100g of L-phenylalanine one by one with a medicine spoon after the addition, After continuing to react in ice bath for 3 hours, neutralize the reaction solution to neutral with ammonia water, and place it in ice bath overnight, and filter to obtain L-4-nitrophenylalanine, m.p.241.5-242.4°C.
Embodiment 3
[0019] Embodiment 3, a kind of synthetic method of L-2,4-dinitrophenylalanine:
[0020] Put a 250mL three-neck flask in an ice bath, add 10g of L-phenylalanine and 95mL of concentrated sulfuric acid, stir to dissolve them completely, add 30g of urea nitrate one spoon at a time, and gradually raise the temperature to 50°C after the addition is complete. Reaction at high temperature for 10h. After the reaction is completed, place it in an ice bath, neutralize the reaction solution to neutrality with sodium hydroxide solution, then remove water on a rotary evaporator, recrystallize the dried solid with methanol, and filter off insoluble inorganic salts. The resulting methanol solution was placed in an ice bath for 12 hours to crystallize, filtered and dried to obtain 12.6 g of L-2,4-dinitrophenylalanine with a yield of 84.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com