Use of a first house dust mite group 2 allergen for treating allergy to a second house dust mite group 2 allergen
A technology for allergens and allergies, applied in allergic diseases, allergen antigen components, animal/human peptides, etc., can solve life-threatening, multiple reactions, allergic side effects and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0045] The preparation of vaccines is generally well known in the art. Allergens can be suitably mixed with excipients that are medicinal and also compatible with the active ingredient. Examples of suitable excipients are water, saline, dextrose, glycerol, ethanol, etc., and combinations thereof. The vaccine may additionally contain other substances such as wetting agents, emulsifying agents, buffering agents or adjuvants to enhance the effect of the vaccine.
[0046] Vaccines can be appropriately formulated with the following excipients commonly used in such formulations, such as pharmaceutical grades of mannitol, lactose, starch, magnesium stearate, sodium saccharin, cellulose, magnesium carbonate, and the like.
[0047] The vaccine should be administered in a manner that matches the dosage formulation and in an amount that is therapeutically effective and immunogenic. The amount of active ingredient included in the vaccine depends on the subject to be treated (ie, the abi...
Embodiment 1
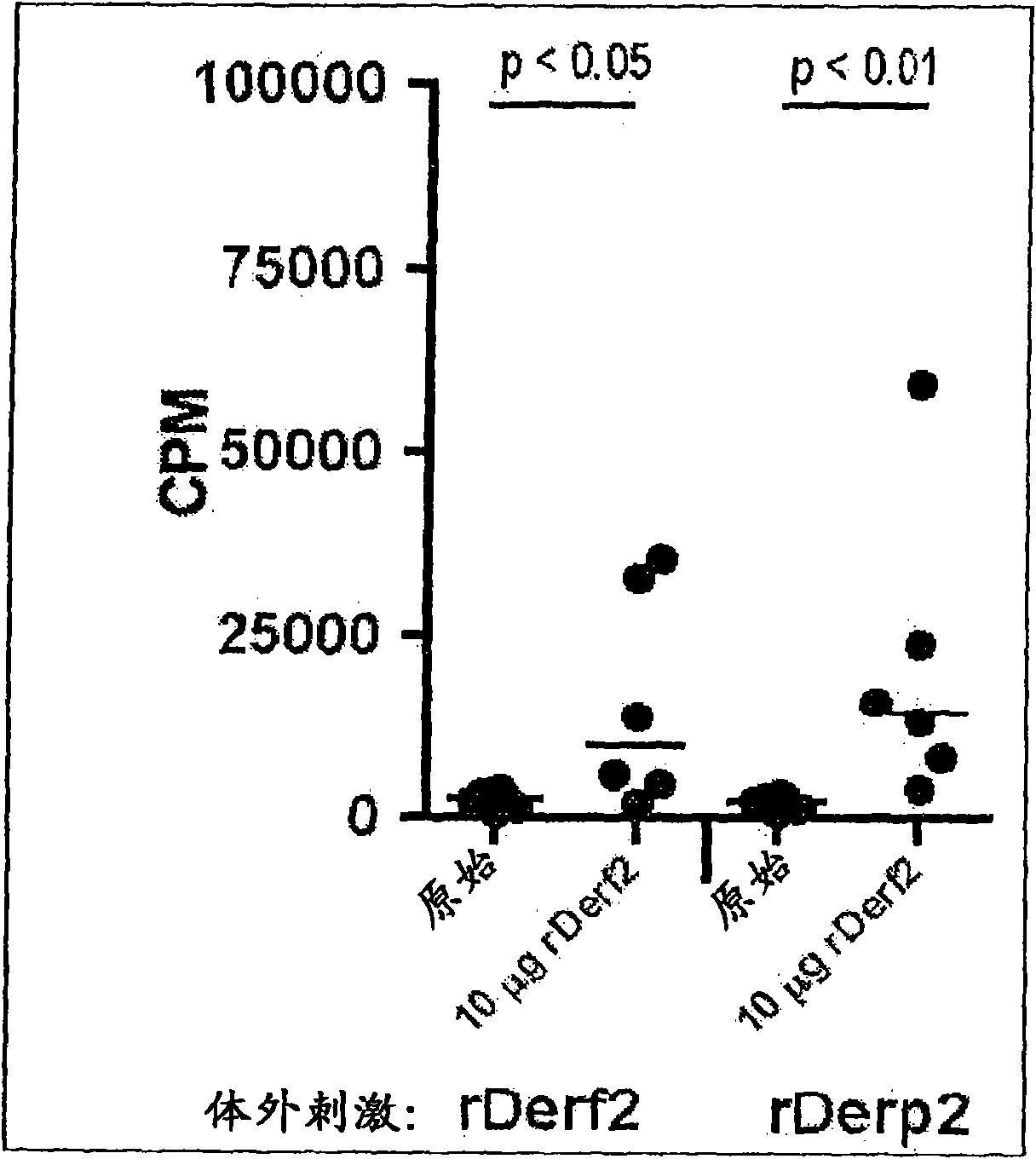
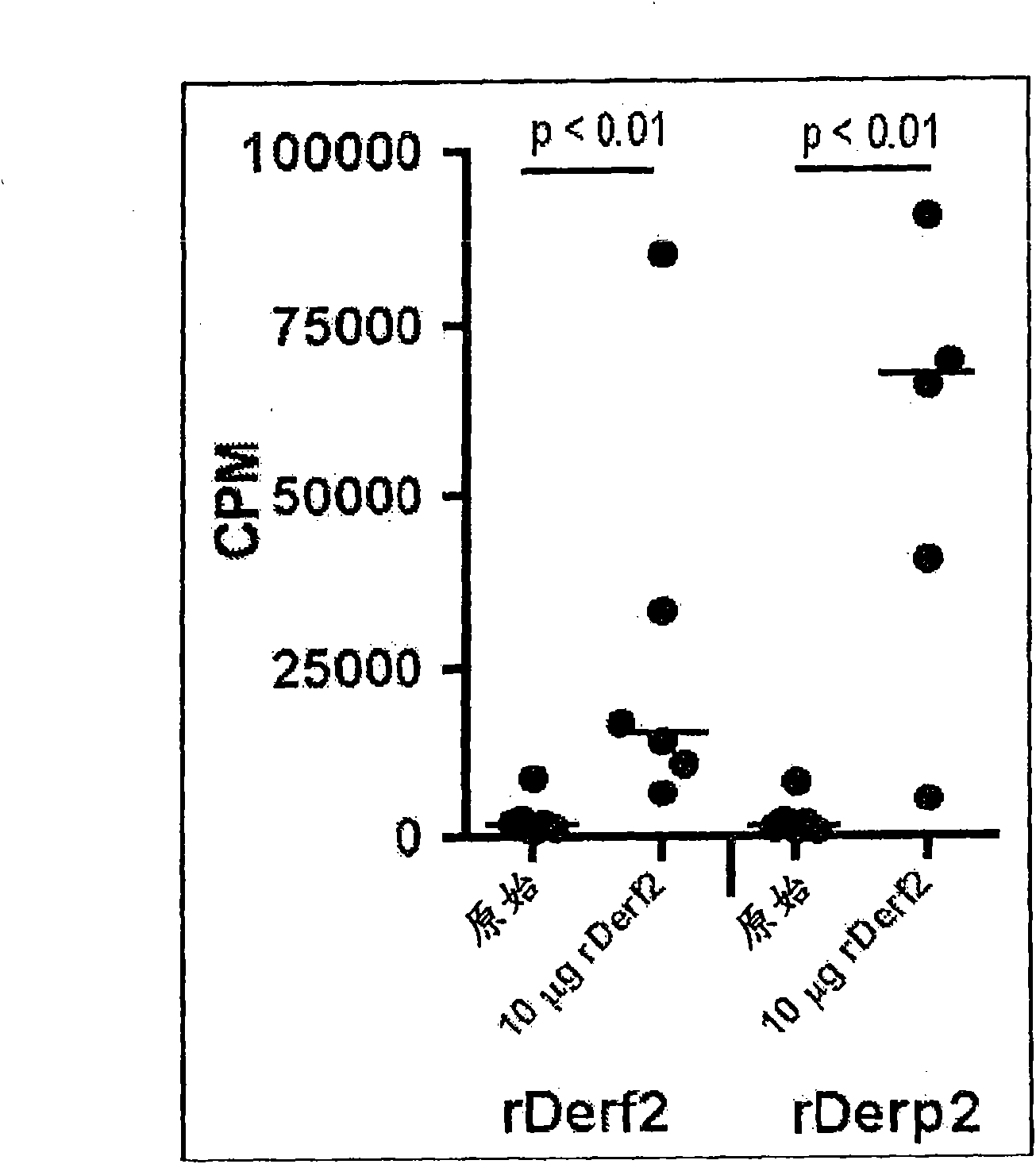
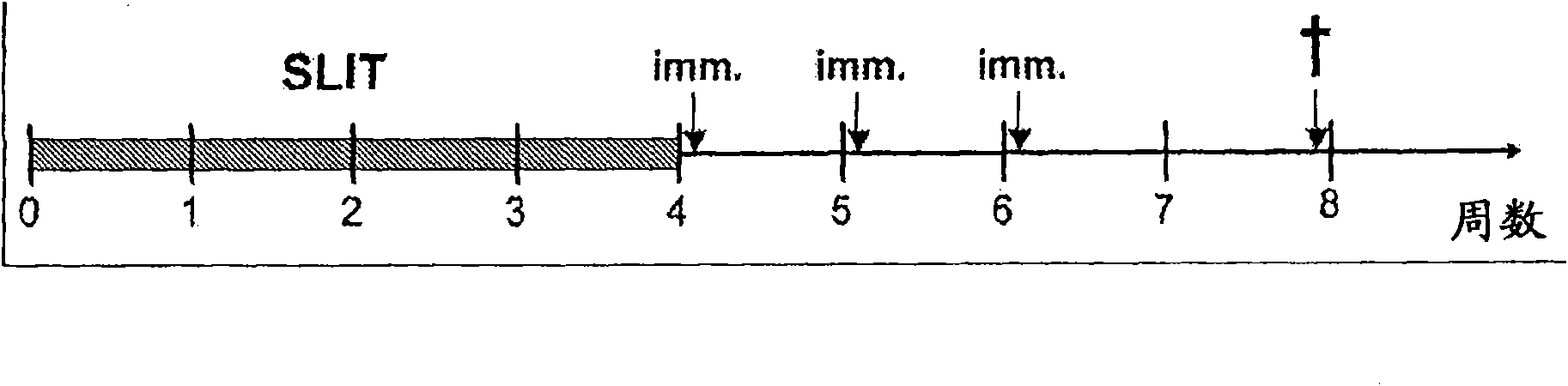
[0082] Example 1: SLIT treatment with recombinant DER F2 and DER P2
[0083] Methods and Materials:
[0084] Expression and purification of rDer f2 and rDer p2 from E. coli
[0085] 用PCR反应扩增编码Der f2和Der p2的cDNA,所使用的引物为derp2DOndeI:gcgcgccatatggatcaagtcgatgtcaaag,derp2UPxhoI:gcgcgcctcgag ttaatcgcggattttagcatg,derf2DOndeI:gcgcgccatatggatcaggtcgatgtcaaag,derf2UPxho1:gcgcgcctcgag ttaatcgcggattttagcgtg,且以携带Der f2和Der p2的cDNA的质粒( pCo06 and pCo10) as templates. The PCR product was cloned into the pETDuet-1 vector, resulting in an additional N-terminal methionine. This vector was introduced into E. coli strain BL21(DE3). Expression of rDer f2 and rDer p2 proteins was induced by adding 1 mM isopropyl-β-thiogalactoside (IPTG) and incubating overnight at 37°C. The purified inclusion bodies were dissolved in 8M urea, 20mM Tris pH8.5 overnight. Proteins were refolded by rapidly diluting to a final concentration of 0.65M urea and incubating for 1 hour at room temperature. Precipitate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com