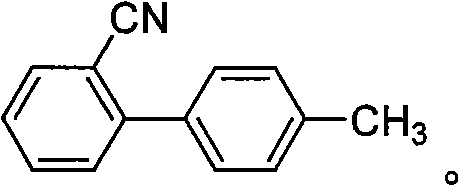
Synthesis method of 4'-Bromomethyl-2-cyanobiphenyl
A technology of bromomethylbiphenyl and synthesis method, applied in the field of synthesis of 2-cyano-4'-bromomethylbiphenyl, achieving the effects of environmental protection, low cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
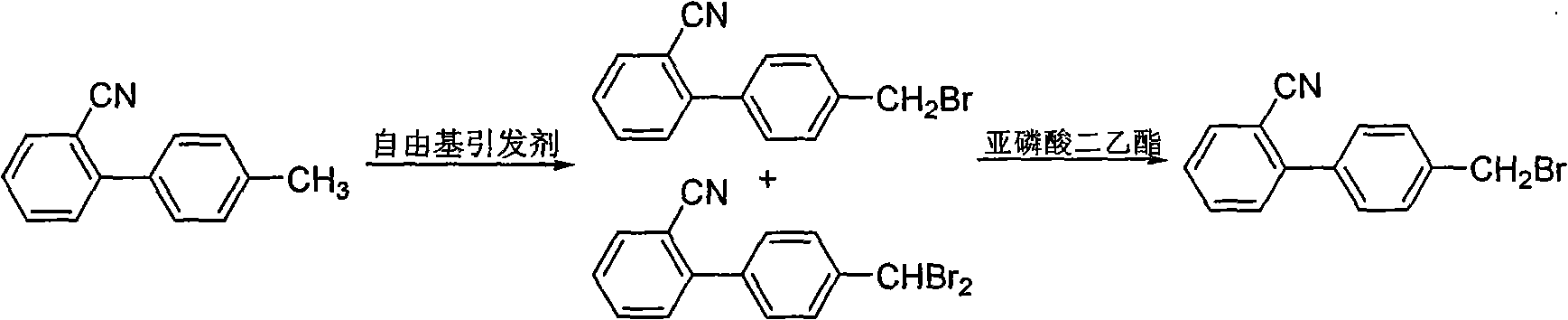
[0028] Embodiment 1: Preparation of 2-cyano-4'-bromomethylbiphenyl, the steps are as follows:
[0029] 1) Add 771g of dichloromethane (10mL / g), 58g of 4'-methyl-2-cyanobiphenyl (1.0eq), 0.5g of 2,2'-azobisisobutyronitrile to a 2L reaction flask in sequence (0.01eq) and 80.1g bromosuccinimide (1.5eq), the bromination reaction was carried out for 4h at a temperature of 45-55°C and a flow rate of 5L / s nitrogen;
[0030] 2) After the reaction solution was lowered to normal temperature, press-filtered, then added 49.7g of diethyl phosphite (1.2eq) to the filtrate, and reacted at 25-35°C for 10h;
[0031] 3) Concentrate the reaction solution under reduced pressure at a temperature of 45-55°C, pump in 405g of pure water (7mL / g) for crystallization once, and obtain 2-cyano-4'-methyl bromide after separation The base biphenyl product was 70.3g, the yield was 86%, and the detection showed that the liquid chromatography purity (HPLC) was 98%. The hydrogen nuclear magnetic spectrum data...
Embodiment 2
[0032] Embodiment 2: Preparation of 2-cyano-4'-bromomethylbiphenyl, the steps are as follows:
[0033] 1) Add 617g of dichloromethane (12mL / g), 39g of 4'-methyl-2-cyanobiphenyl (1.0eq), and 3.3g of 2,2'-azobisisobutyronitrile in sequence in a 2L reaction flask (0.1eq) and 35.6g bromosuccinimide (1.0eq), the bromination reaction was carried out for 5h at a temperature of 45-55°C and a flow rate of 10L / s nitrogen;
[0034] 2) After the reaction solution was lowered to normal temperature, press filter, then add 27.6g of diethyl phosphite (1.0eq) to the filtrate, and react at 25-35°C for 14h;
[0035] 3) Concentrate the reaction solution under reduced pressure at a temperature of 45-55°C, pump in 464g of pure water (12mL / g) for crystallization once, and obtain 2-cyano-4'-methyl bromide after separation Base biphenyl product 43g, yield 82%, detection shows that liquid chromatography purity (HPLC) is 98%. The hydrogen nuclear magnetic spectrum data (1H-NMR) is: (300MHZ, CDCl3), δ4...
Embodiment 3
[0036] Embodiment 3: Preparation of 2-cyano-4'-bromomethylbiphenyl, the steps are as follows:
[0037] 1) Add 925g of dichloromethane (18mL / g), 39g of 4'-methyl-2-cyanobiphenyl (1.0eq), 6.5g of 2,2'-azobisisobutyronitrile in sequence in a 2L reaction flask (0.2eq) and 78g bromosuccinimide (2.2eq), carry out the bromination reaction for 4.5h at a temperature of 45-55°C and a flow rate of 8L / s nitrogen gas
[0038] 2) After the reaction solution was lowered to normal temperature, press filter, then add 69g of diethyl phosphite (2.5eq) to the filtrate, and react at 25-35°C for 11h;
[0039]3) Concentrate the reaction solution under reduced pressure at a temperature of 45-55°C, pump in 695g of pure water (18mL / g) for crystallization once, and obtain 2-cyano-4'-methyl bromide after separation Base biphenyl product 40g, yield 81%, detection shows that liquid chromatography purity (HPLC) is 98%. The hydrogen nuclear magnetic spectrum data (1H-NMR) is: (300MHZ, CDCl3), δ4.56 (methyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com