Aryl sulfamide derivatives and methods of their use
A technology of heteroaryl and alkyl, applied in the field of arylsulfonamide derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0939] Example 1 : 3-[3-(4-chlorophenyl)-2,2-dioxo-2,1,3-benzothiadiazole-1(3H)-yl]-N-methylpropan-1-amine
[0940]
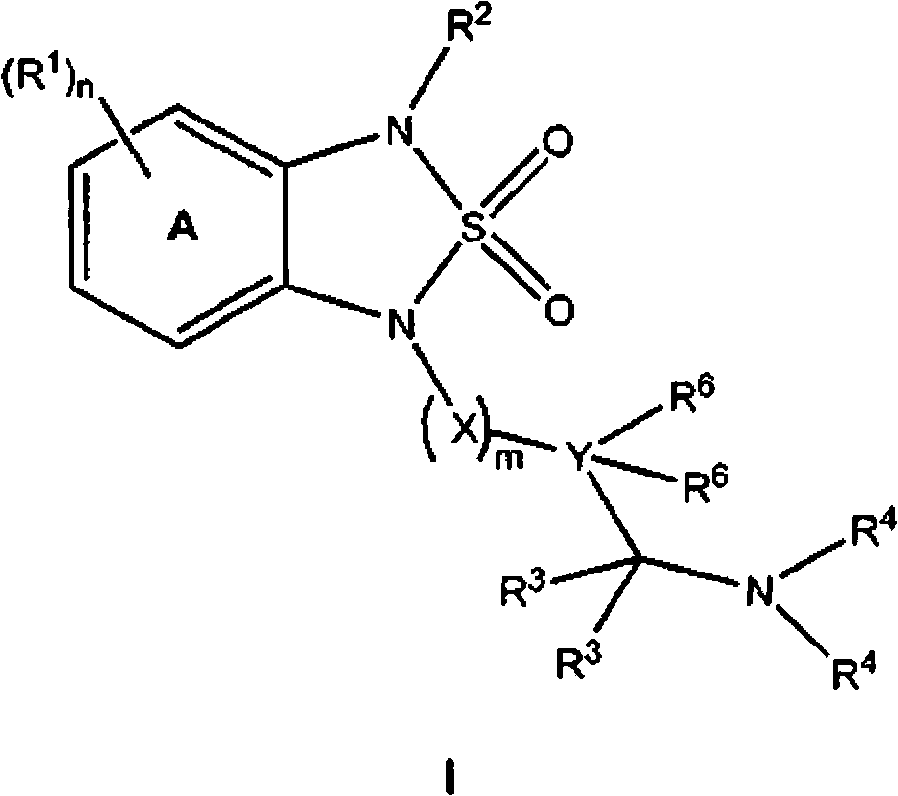
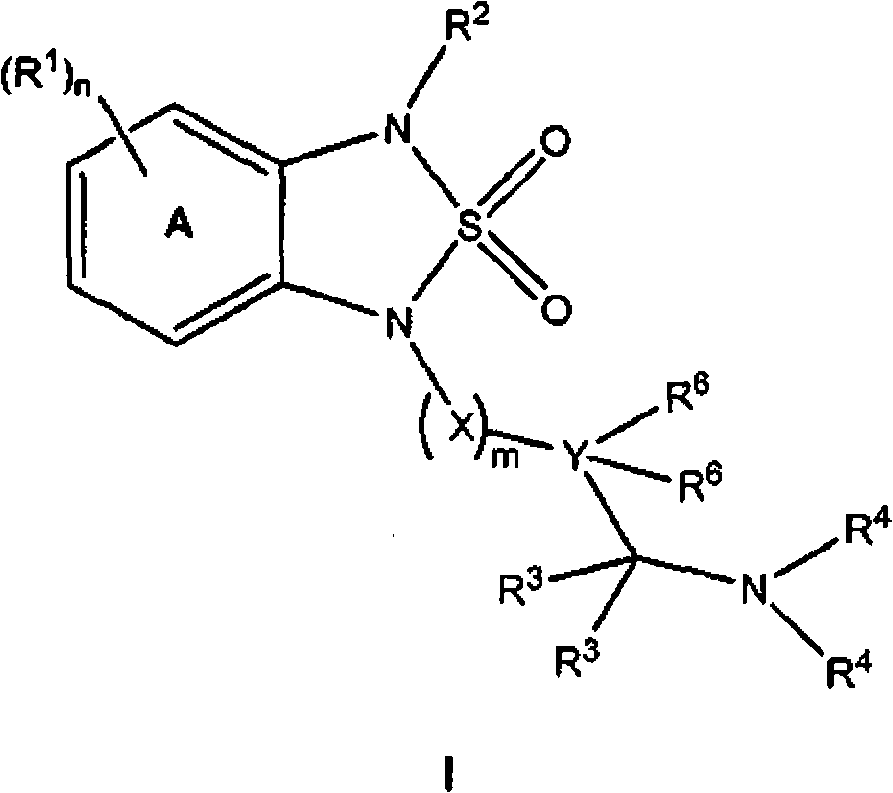
[0941] General procedure A for the synthesis of sulfonamides of structure I:
[0942] step 1 : Under a nitrogen atmosphere, anhydrous diglyme (10 mL) was added to a flask equipped with a dropping funnel and allowed to reflux vigorously. Dissolve N-(4-chloro-phenyl)-benzene-1,2-diamine (1.09g, 5.0mmol) and sulfonamide (0.58g, 6.0mmol) in 5mL diglyme and Put it in the dropping funnel. The mixture was added dropwise to the flask over 15 minutes, and then reflux was continued for another 15 minutes. The mixture was cooled to ambient temperature and diluted with ether, washed with water, 2N HCl, water, brine, dried over anhydrous magnesium sulfate and concentrated. The crude product was purified via Iscochromatography (Redisep, silica, 5-50% (ethyl acetate containing 2% formic acid) gradient in hexane) to obtain 1-(4 -Chlorophenyl)-1,3-dihydro-2,1,3-benzothiadiazol...
example 2
[0949] Example 2 : 3-[3-(4-chlorophenyl)-2,2-dioxo-2,1,3-benzothiadiazole-1(3H)-yl]propan-1-amine
[0950]
[0951] The 1-(3-bromo-propyl)-3-(4-chloro-phenyl)-1,3-dihydrobenzo[1,2,5]thiadiazole 2,2-dioxide (0.10 g, 0.25 mmol) was dissolved in 7N methylamine (20 mL) in methanol, heated to 60°C and stirred in a sealed flask for 16 hours. The mixture was cooled and concentrated in vacuo to give the crude product. The crude product was purified via chromatography (silica, 5% methanol saturated with ammonia in chloroform) to give 73 mg (87%) 3-[3-(4-chlorophenyl)-2,2-dioxo-2,1,3-benzene Thiadiazole-1(3H)-yl)prop-1-amine . The free base was dissolved in ether (2 mL) and treated with 1N hydrochloric acid (1 equivalent) in ether. The white precipitate was collected, dissolved in water, and lyophilized to obtain 56 mg 3-[3-(4-chlorophenyl)-2,2- Dioxo-2,1,3-benzothiadiazole-1(3H)-yl]prop-1-amine hydrochloride.
[0952] MS(ES)m / z 337.9;
[0953] HPLC purity: 100% at 210-370nm, 7.7 minutes...
example 3
[0954] Example 3 : N-{3-[3-(4-chlorophenyl)-2,2-dioxo-2,1,3-benzothiadiazole-1(3H)-yl]propyl}cyclopropylamine
[0955]
[0956] With general procedure A Step 3 In a similar way, 1-(3-bromo-propyl)-3-(4-chloro-phenyl)-1,3-dihydrobenzo[1,2,5]thiadiazole 2 is treated with cyclopropylamine, 2-Dioxide (0.10g, 0.25mmol), providing N-{3-[3-(4-chlorophenyl)-2,2-dioxo-2,1,3-benzothiadiazole- 1(3H)-yl]propyl}cyclopropylamine (87 mg).
[0957] MS(ES)m / z 378
[0958] HPLC purity: 100% at 210-370nm, 8.2 minutes; Stella RP18, 3.5u, 150×4.6mm column, 1.2mL / min, 85 / 15-5 / 95 (ammonium formate buffer, pH=3.5, Acetonitrile / MeOH) lasted 10 minutes and held for 4 minutes.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com