In vitro amplification method for regulatory T cells
An in vitro amplification and regulatory technology, applied in the field of biomedicine, can solve the problems of prolonging the survival of allografts, failure to achieve tolerance, prolonging survival time, etc., and achieve the effect of large clinical application value and sufficient quantity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
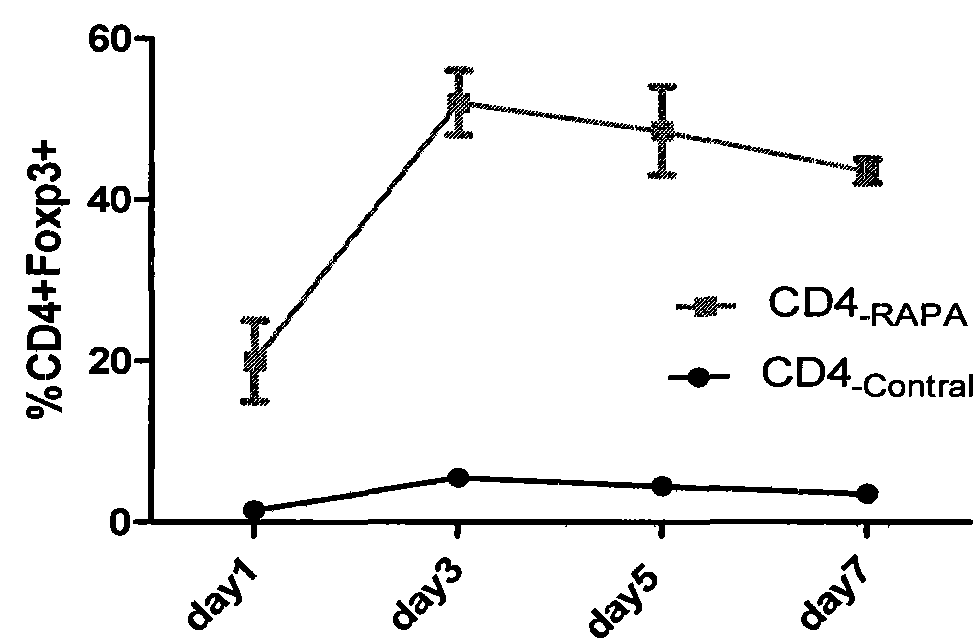
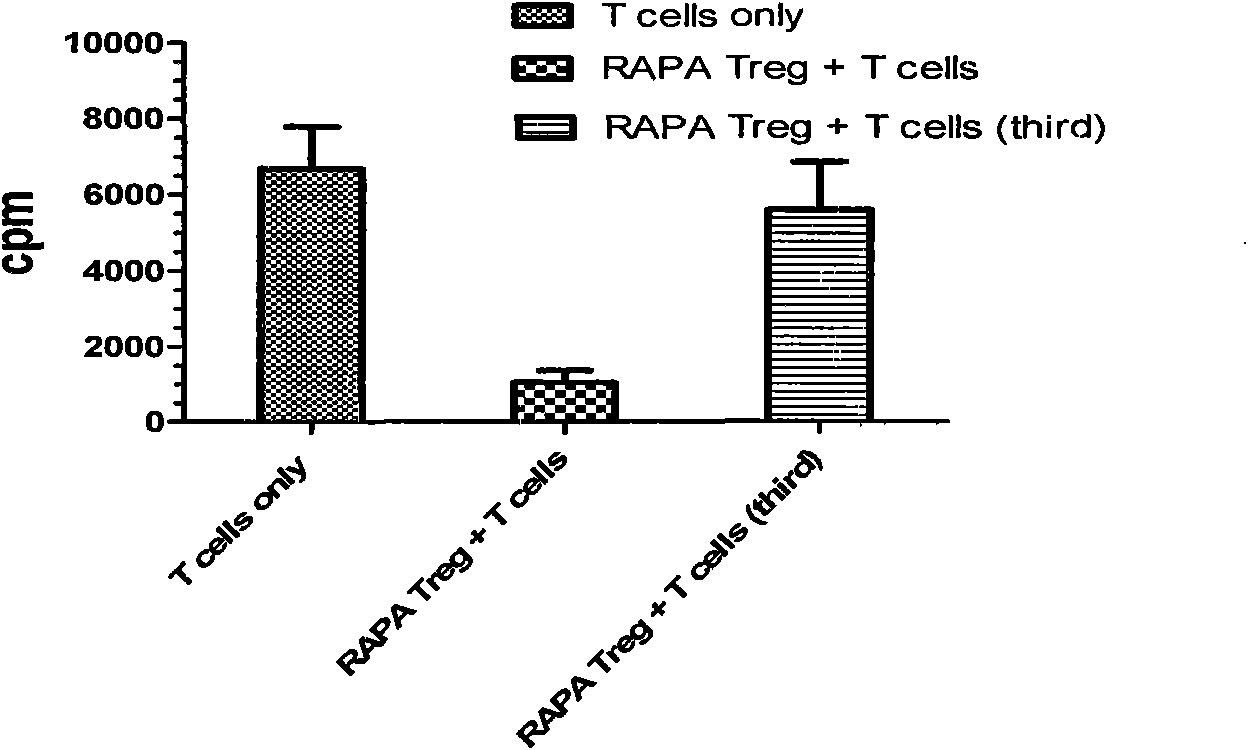
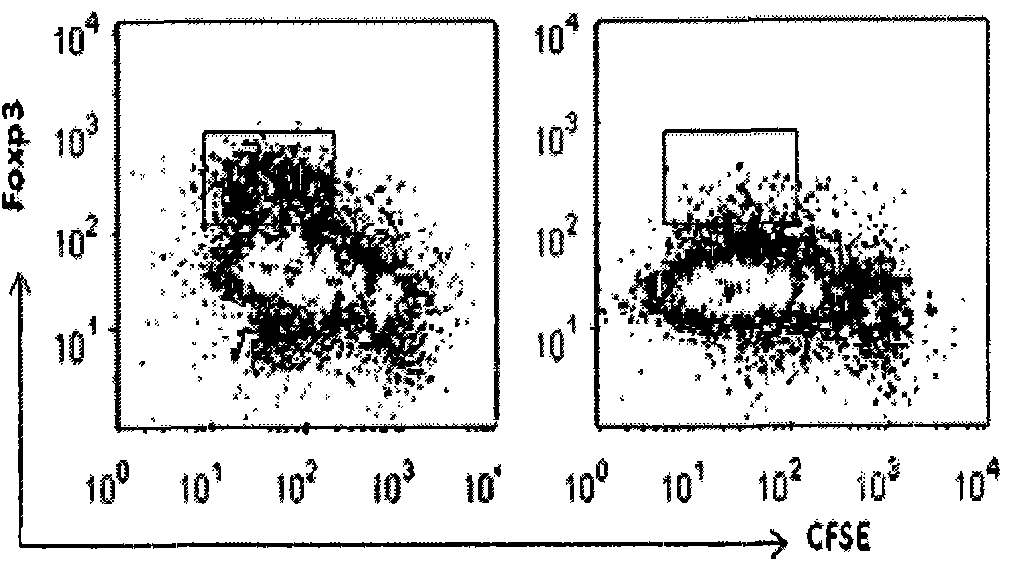
[0031] Materials: CD4+ T cell isolation kit (purchased from Miltenyi Biotec, USA), anti-human CD3 (BD Com., USA) coated culture plate (purchased from VWR, USA), systemic immunodeficiency (SCID) mice (purchased from Jackson Laboratory, USA).
[0032] Instruments: magnetic bead separator (Auto MACS from Miltenyi, Germany), flow cytometer (BD company, model: Vantage SE).
[0033] Reagent: Inducer: anti-CD3CD28-beads (1:10 ratio to cells, Invitrogen), rhIL-2 (final concentration after addition is 100IU / mL), rhTGF-β (final concentration after addition is 5ng / mL) Purchased from BD Company of the United States and Rapamycin (final concentration after adding was 10nM) was purchased from Wyeth Pharmaceuticals. Amplifier: rhIL-2 (20IU / mL), anti-humanCD28 (1ng / ml), Rapamycin (10nM, Wyeth Pharmaceuticals); culture medium was supplemented with 100U / ml penicillin in complete RPMI-1640 medium, 100μg / ml chain Mycin, 2mM L-glutamic acid, 10mM 4-hydroxyethylpiperazineethanesulfonic acid, 0....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com