Ciclesonide aerosol and preparation method thereof
A ciclesonide and aerosol technology, which is applied in the field of ciclesonide aerosol and its preparation, and achieves the effects of great application value, convenient taking and less adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
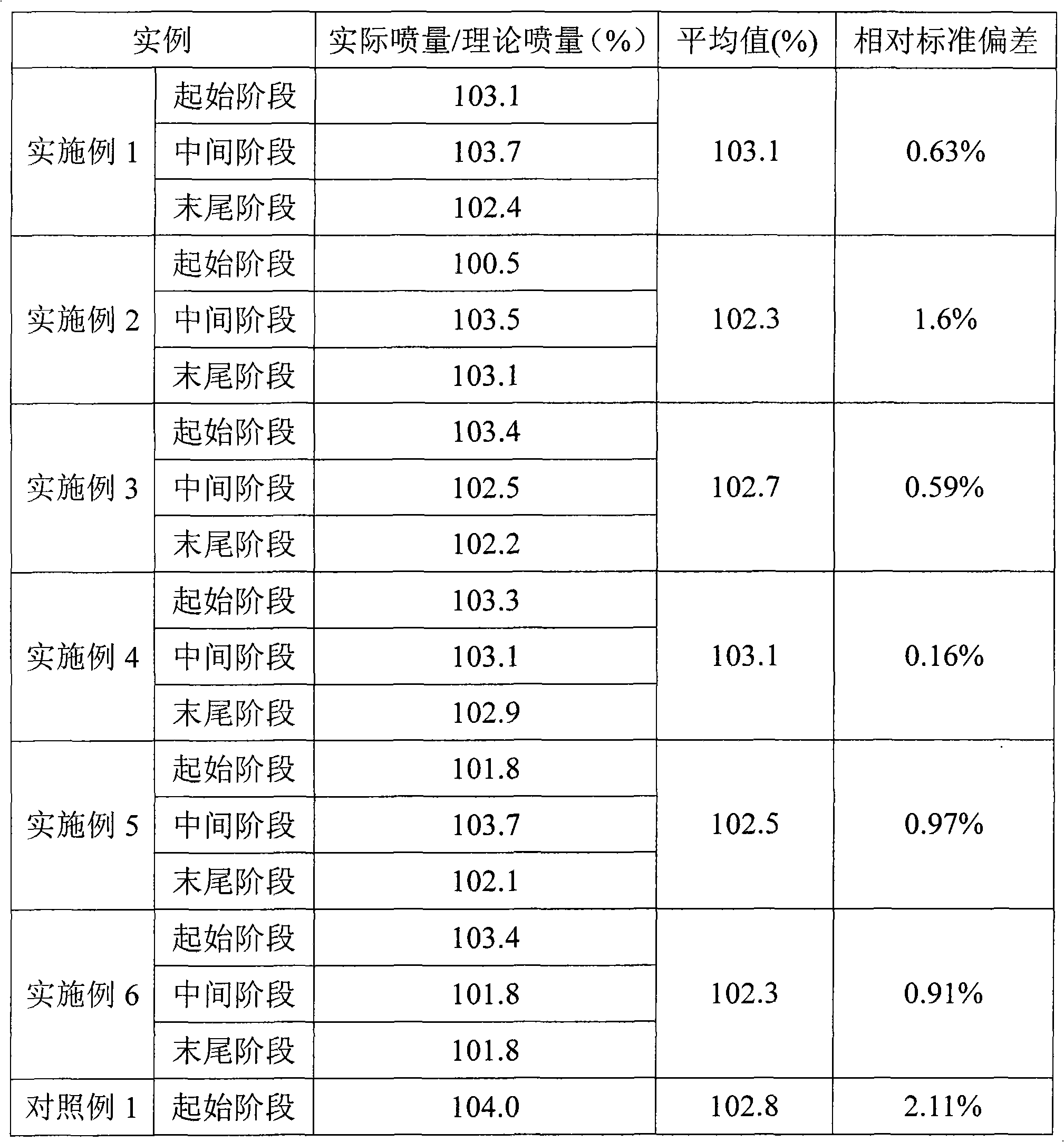
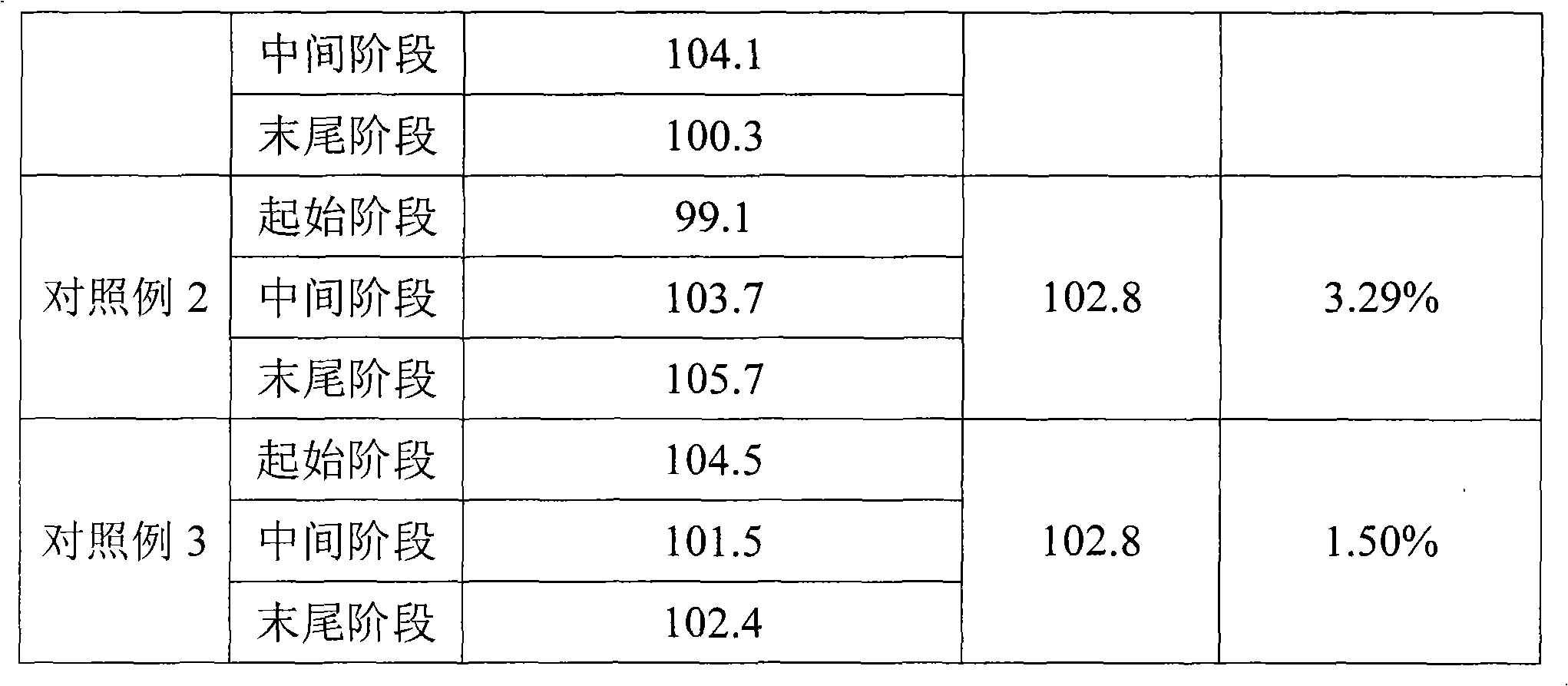
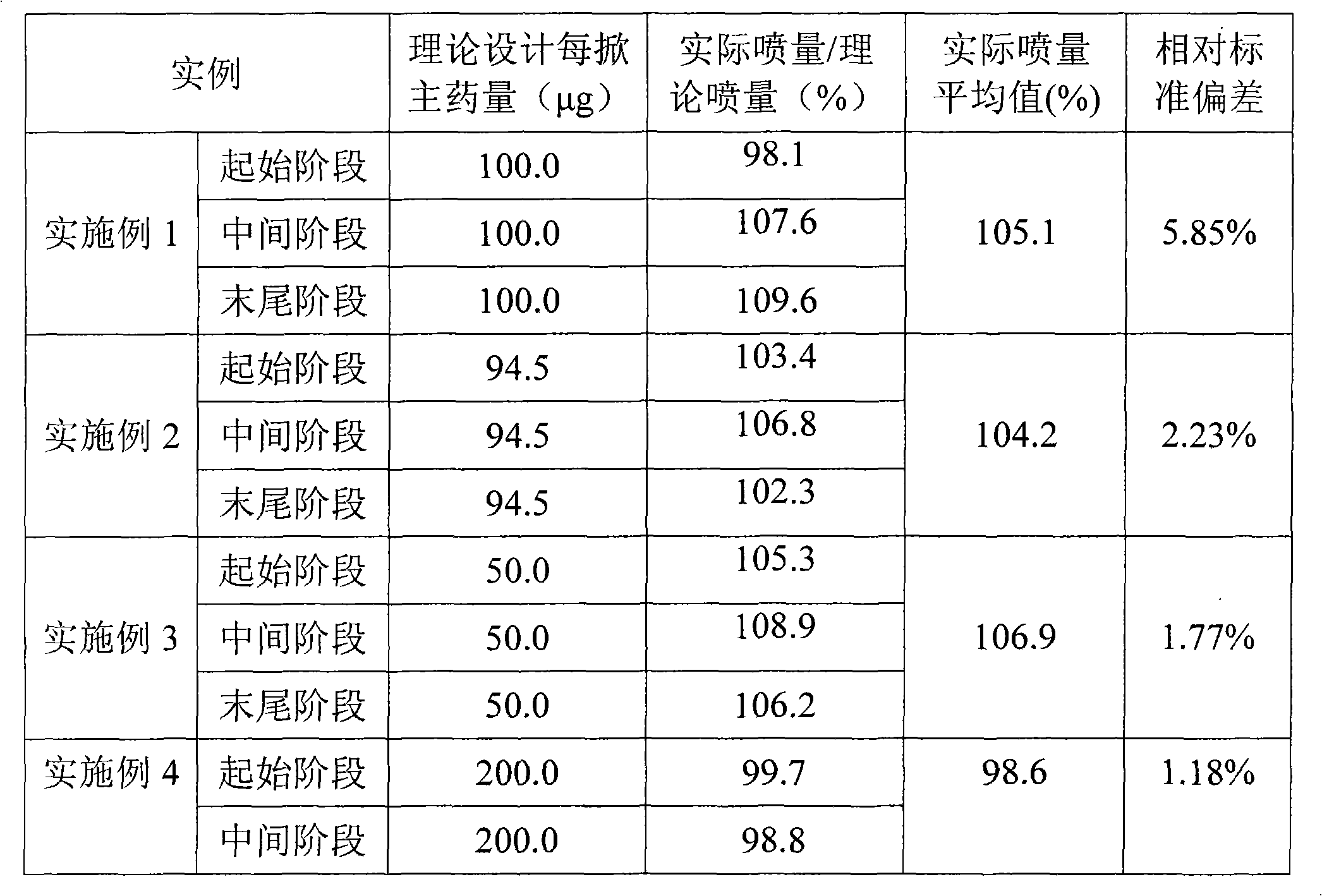
Embodiment 1
[0027] Material amount (g)
[0028] According to the prescribed amount, ciclesonide, lecithin, and absolute ethanol were mixed and stirred to form a solution, and the solution was quantitatively injected into an unsealed aerosol bottle, and the 50 μl quantitative valve was grasped and sealed on the bottle mouth. The propellant HFA 134a is then dosed into aerosol bottles by pressurized filling. Install an actuator with an orifice diameter of 0.4mm. The amount of each spray is 59.63mg, and the amount of main drug per spray is 100.0μg.
Embodiment 2
[0030] Material amount (g)
[0031] Absolute ethanol
[0032] Mix and stir ciclesonide, oleic acid, absolute ethanol, and propellant 134a according to the prescribed amount to form a solution, and quantitatively pour the solution into an aerosol bottle with a 63 μl quantitative valve that has been grasped and sealed by pressurized filling . Install an actuator with an orifice diameter of 0.4mm. The amount of each spray is 76.44mg, and the amount of main drug per spray is 94.5μg.
Embodiment 3
[0034] Material amount (g)
[0035] According to the prescribed amount, ciclesonide and absolute ethanol were mixed and stirred to form a solution, and the solution was quantitatively injected into an unsealed aerosol bottle, and the 50 μl quantitative valve was grasped and sealed on the bottle mouth. The propellant HFA 227 is then dosed into aerosol bottles by pressurized filling. Install an actuator with an orifice diameter of 0.4mm. The amount of each spray is 69.08mg, and the amount of main drug per spray is 50.0μg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com