Replication defective recombinant adenovirus Ad41 vector system and application thereof
A recombinant adenovirus, replication-deficient technology, applied in the direction of virus/phage, application, and cells modified by introducing foreign genetic material, can solve the problem of reducing the infectivity of Ad5, and achieve the effect of broad application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
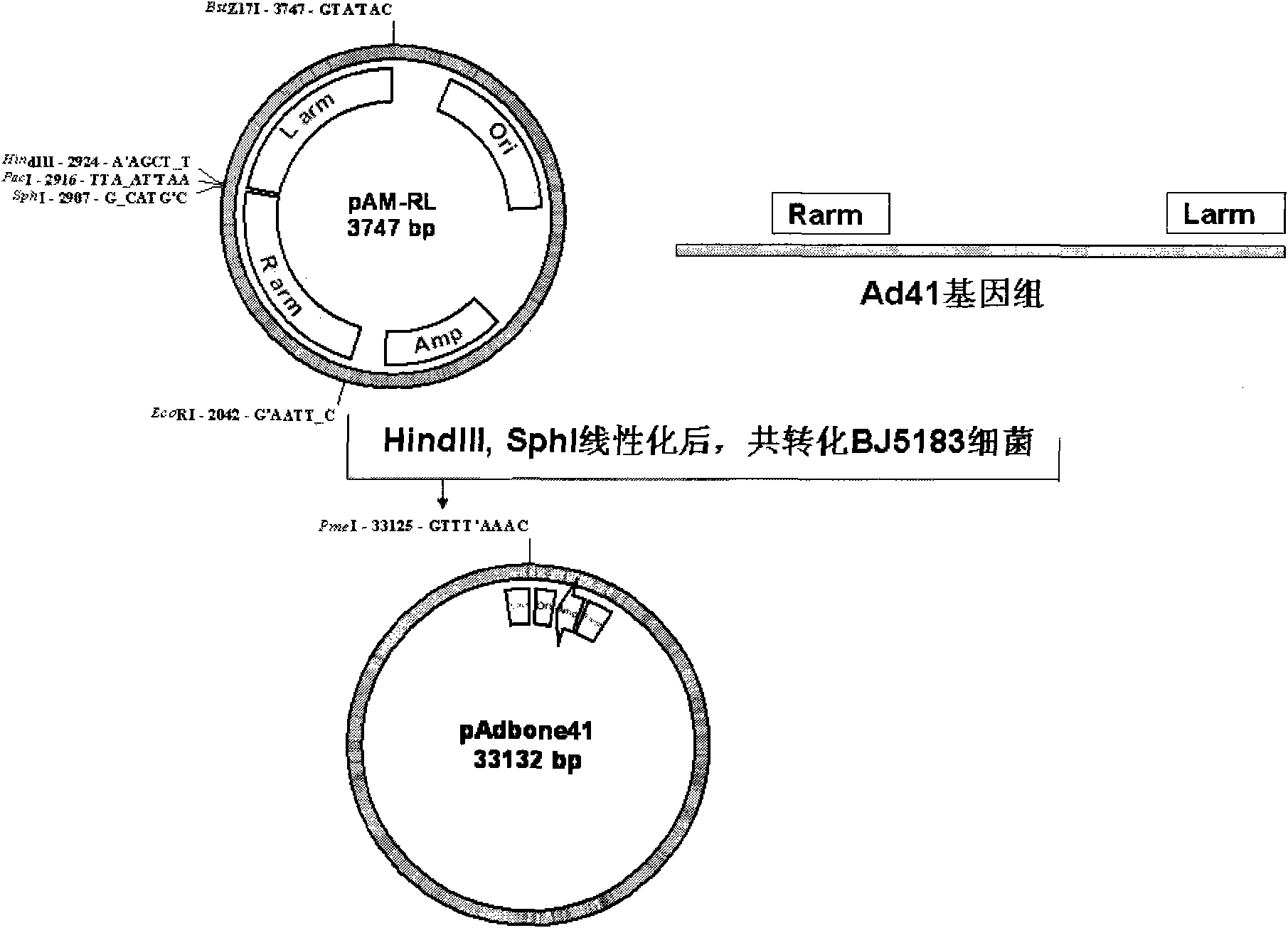
[0023] Embodiment 1, construction of backbone plasmid pAdbone41
[0024] 1) Construction of plasmid pAM-RLarm containing left arm, right arm (L arm, R arm) sequences, replicon and ampicillin resistance gene sequence for recombination
[0025] Under standard polymerase chain reaction (PCR) conditions (50 microliter system: 10 nanograms of template, 30 picomoles of primers, 10 nanomoles of each of the four deoxynucleoside triphosphates, 1 unit of high-fidelity thermostable DNA polymerase ;Reaction conditions: pre-denaturation at 94 degrees Celsius for 2 minutes, denaturation at 94 degrees Celsius for 30 seconds, annealing at a temperature 4 degrees Celsius lower than the melting temperature of the primer for 30 seconds, extension at 68 degrees Celsius, and the extension time is based on the length of the product. Amplify 800 base pairs It takes 1 minute to calculate, 30 cycles of amplification, and 68 degrees Celsius for 5 minutes to fill in the end; the following PCR amplificat...
Embodiment 2
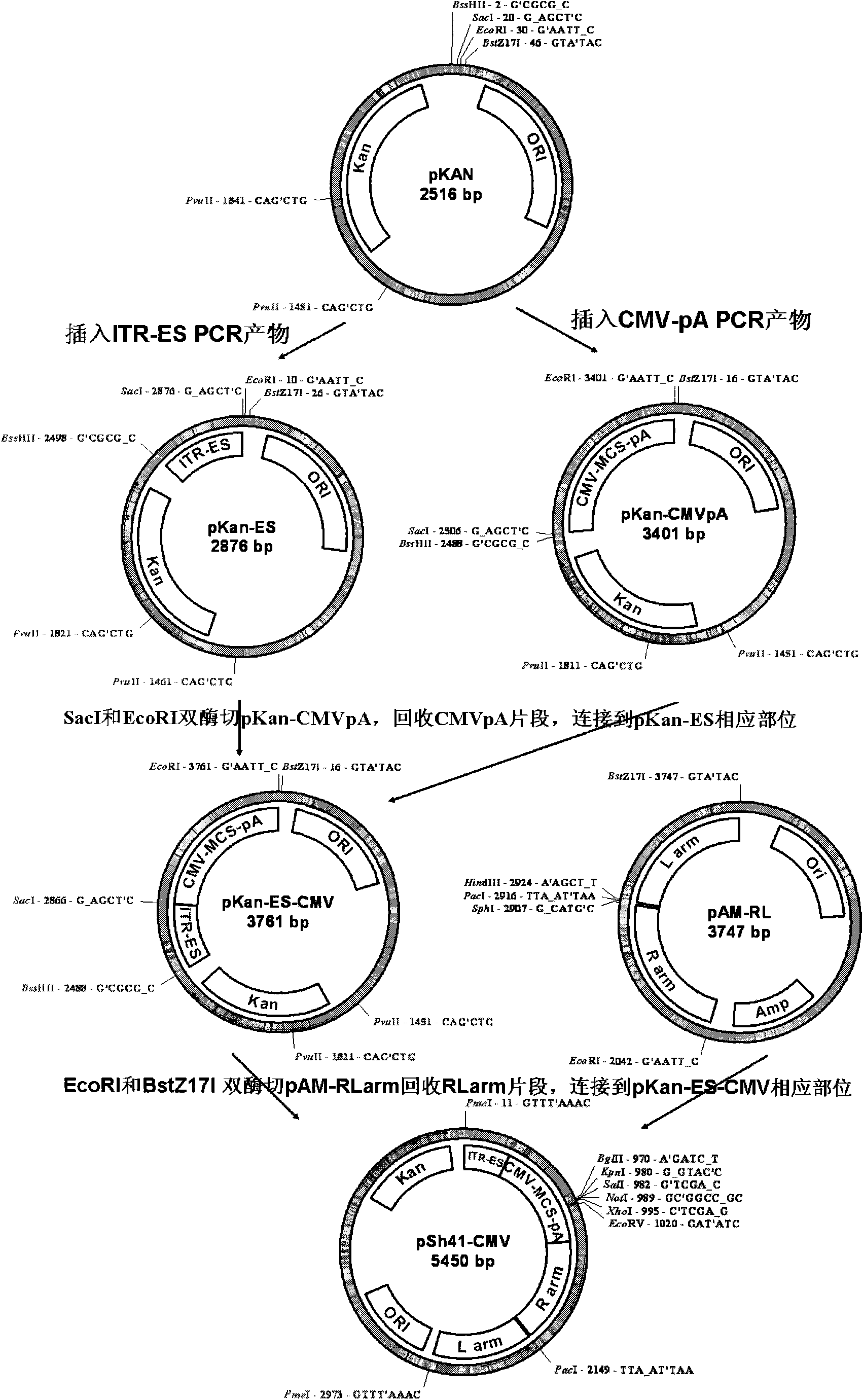
[0029] Embodiment 2, the construction of shuttle plasmid pSh41-CMV ( figure 2 )
[0030] 1) Construction of the cloning plasmid pKan containing the resistance gene (Kan) of kanamycin
[0031] Using pShuttle-CMV (Stratagene Company) as a template, use primer 5F070522: gcccggatcc (BamHI) cgagcggtatcagctcactcaaag (SEQ ID NO: 6) and primer 5R070522: gccccgcgcgc (BssHII) aaactggaacaacactcaaccctat (SEQ ID NO: 7) to amplify the 2483bpt fragment (refer to Schutt - CMV sequence, version017005; fragment position: 4660nt-7122nt), this fragment contains the replication origin and the kanamycin resistance gene. Use endonucleases BamHI and BssHII located at both ends of the fragment to recover after double digestion; primer 5F: cgcgcaaggg gaagagctcg ggaaggggaattcggagaagggtatacc (SEQ ID NO: 8) and primer 5R: gatcggtatacccttctccgaattccccttcccgagctcttccccttg (SEQ ID NO: 9) were mixed and annealed to obtain both ends It is a double-stranded DNA with a length of about 50bp at the cohesive end...
Embodiment 3
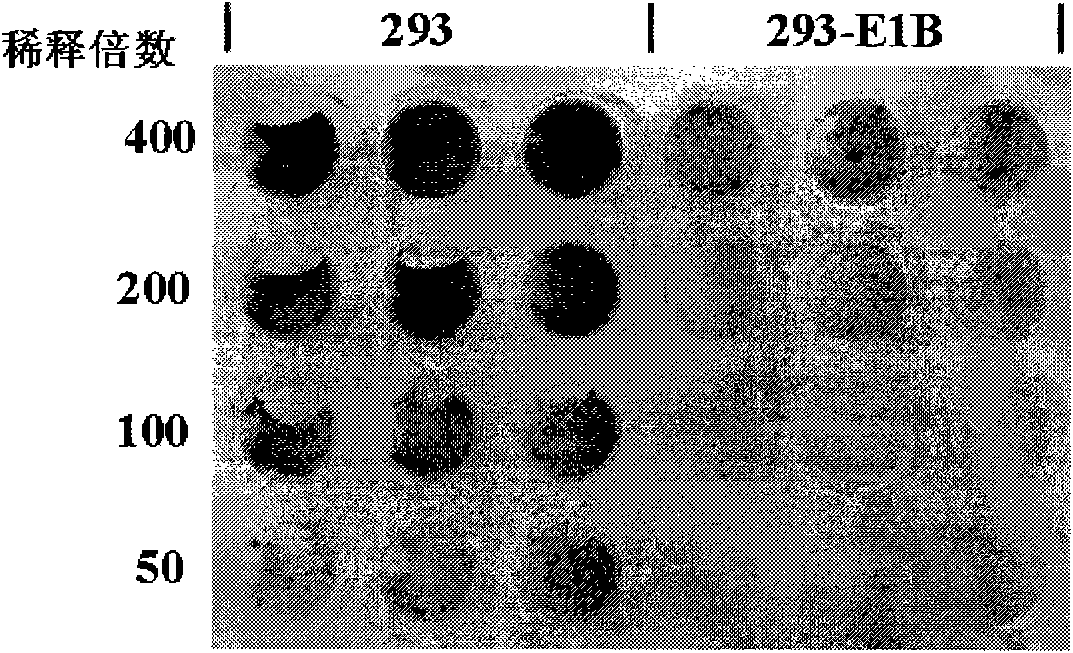
[0038] Example 3, construction of packaging cell line 293-E1B
[0039] 1) Cloning of Ad41 E1B55K gene
[0040] The E1B55K gene was amplified by PCR using the DNA of stool samples from Ad41-positive infants with viral diarrhea as a template, and the reaction conditions were the same as before. The primer used is 8F: gccc aagctt (HindIII) atggagcgcccaaacccatctg (SEQ ID NO: 14) and 8R: gccc tctaga (Xbal) tacccttaatcctcatcgctggattc (SEQ ID NO: 15), amplifies a fragment containing the complete CDS of E1B55K. The amplified fragment was connected to the T vector (pMD-18T, TAKARA company), after sequencing confirmed that the sequence was correct (sequencing was carried out at Beijing Aoke Biotechnology Company), it was digested with HindIII and XbaI, and connected to the pcDNA3 plasmid (Invitrogen). Between the restriction sites HindIII and XbaI, the eukaryotic expression plasmid pcDNA3-E1B55K was obtained.
[0041]2) Gene transfection and screening of cell lines
[0042] The p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com