Method for preparing high-sensitivity immunity quantitative latex testing reagent
An immunoquantitative and sensitive technology, applied in biological tests, measuring devices, material testing products, etc., can solve the problems of insufficient sensitivity, inability to form immune binding networks, and inability of protein molecules to improve sensitivity and increase secondary immune responses. speed, and the effect of improving the sensitivity of the reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
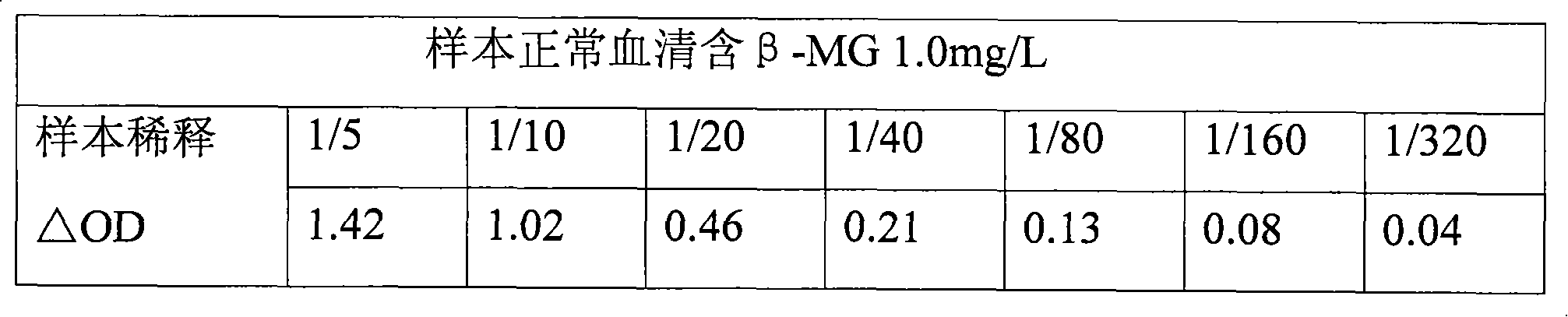
[0015] (1) Preparation of Carrier Immunoquantitative Latex Microparticles Pure water is heated to boiling to remove most of the oxygen in the water. After cooling, take 750ml and add 1.0g sodium dodecylsulfonate SDS to keep the temperature at 65°C for 10 minutes. After the SDS is in a suspended solution state, add 30ml of styrene monomer with a content greater than 98%, then raise the temperature to 75°C, and add 5.2ml of acrylic acid (pH adjusted to 5.5-6.5) after equilibrating for 15 minutes, and then add potassium persulfate after equilibrating. Start timing and gradually raise the temperature to 84°C. The reaction time is 30 minutes. Add neutralized acrylic acid in two times according to 4 times the amount of carboxyl groups on the surface of the microparticle size until the surface of the microspheres is fully carboxylated. Aging at 84°C for two hours , natural cooling. Calculate the concentration of the reaction based on the effective amount added to the reaction and the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com