Immune agent for controlling and curing I type diabetes mellitus
A technology of immunological preparations and diabetes, applied in the field of immunological preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1 prepares immune preparation SUP-NKT
[0039] 1 Materials and methods:
[0040] 1.1 Materials
[0041] C57BL / J mice (female, 18-20 g in weight, 6-8 weeks old): from the third-level animal room of the Experimental Animal Center of the PLA General Hospital;
[0042] RPMI1640 medium: purchased from Gibco;
[0043] MTT dye (tetramethylazolium salt 3-(4.5-dimethyliazol-zyl)-2.5-diphenyl tetrazoliumbromide): purchased from Sigma;
[0044] Dimethylsulfoxide (DMSO): purchased from Solarbio;
[0045] Fetal bovine serum: purchased from Veterinary Prevention and Control Center of Beijing Military Region;
[0046] SEB: prepared according to the method of Chinese patent ZL01103991.4;
[0047] Anti-CD3-PerCP, CD69-FITC, CD8-PE, NK1.1-APC fluorescent antibodies: all purchased from BD Company;
[0048] IL-2: purchased from Beijing Sihuan Biopharmaceutical Co., Ltd.
[0049] 1.2 Method
[0050] 1.2.1 Normal mouse lymphocyte preparation
[0051] Extract the splenocyt...
Embodiment 2
[0054] The active ingredient identification of embodiment 2 immune preparation SUP-NKT
[0055] Method: Analysis of SEB-activated SUP-NKT cell subsets
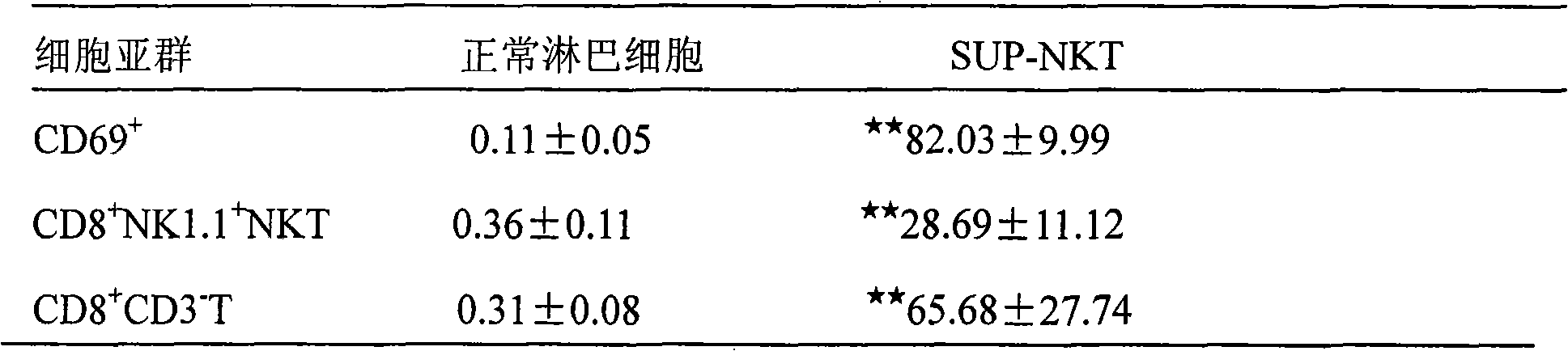
[0056] Add 1-10 × 10 in a 96-well plate 5 Cells (100 μl / well) were added with 0.1 mL SEB (200-400 μg / L), and cultured in a 37° C., 50 mL / L CO2 incubator. SUP-NKT cultured on days 10-30 were stained with anti-CD3-PerCP, CD69-FITC, CD8-PE and NK1.1-APC fluorescent antibodies, and the NKT cells were determined by flow cytometry (FACs Calibue BD, USA). The percentage of T cell subset proliferation and the differentiation pathway of the two cell subsets were recorded.
[0057] The results are shown in Table 1 and Table 2:
[0058] The main components of Table 1 SUP-NKT (n=4)
[0059]
[0060] t-test, ★★ P★ P<0.05 compared with C57BL / J group
[0061] Table 210-The composition of SUP-NKT within 30 days does not change (n=3~4)
[0062]
[0063] t-test, ★★ P<0.01, compared with C57BL / J group
[0064] The above results sh...
Embodiment 3
[0065] Example 3 Identification of SUP-NKT in vivo control and treatment of type 1 diabetes
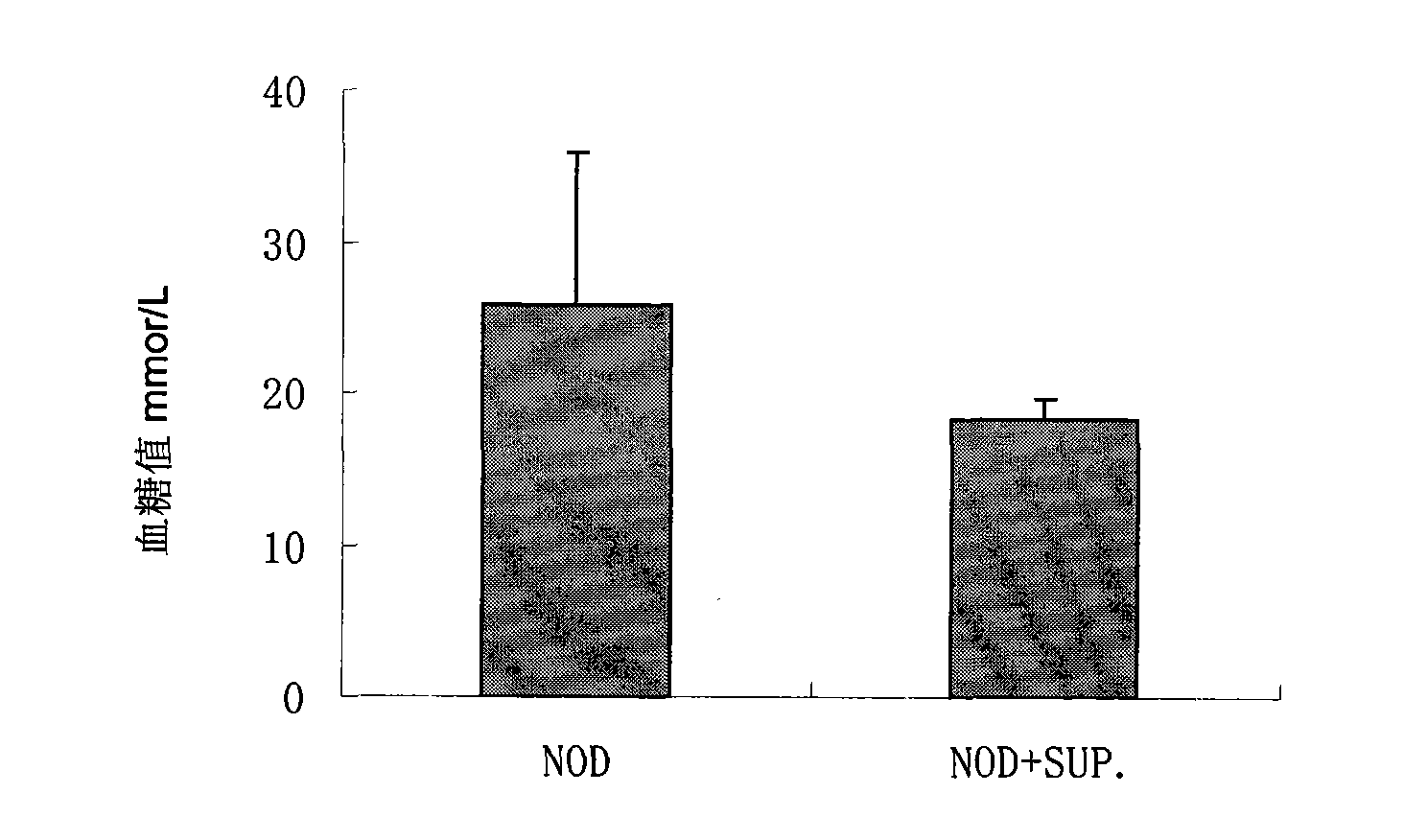
[0066] Experimental animals and experimental methods: two mice with different MHC genetic backgrounds were selected for cell transplantation. The recipients were type I diabetes genetic mice NOD mice (female, raised in the experimental level 2 animal room), and the donors were SUP-NKT from C57BL / J mice. Use a blood glucose meter and a urine sugar meter to determine the outcome of the disease. Experimental animals were grouped as follows:
[0067] 1) NOD group (control group):
[0068] Ten NOD mice aged 3-5 weeks were intraperitoneally injected with normal saline and raised in a second-level animal room. They waited for the natural onset and recorded the incidence within 22 weeks.
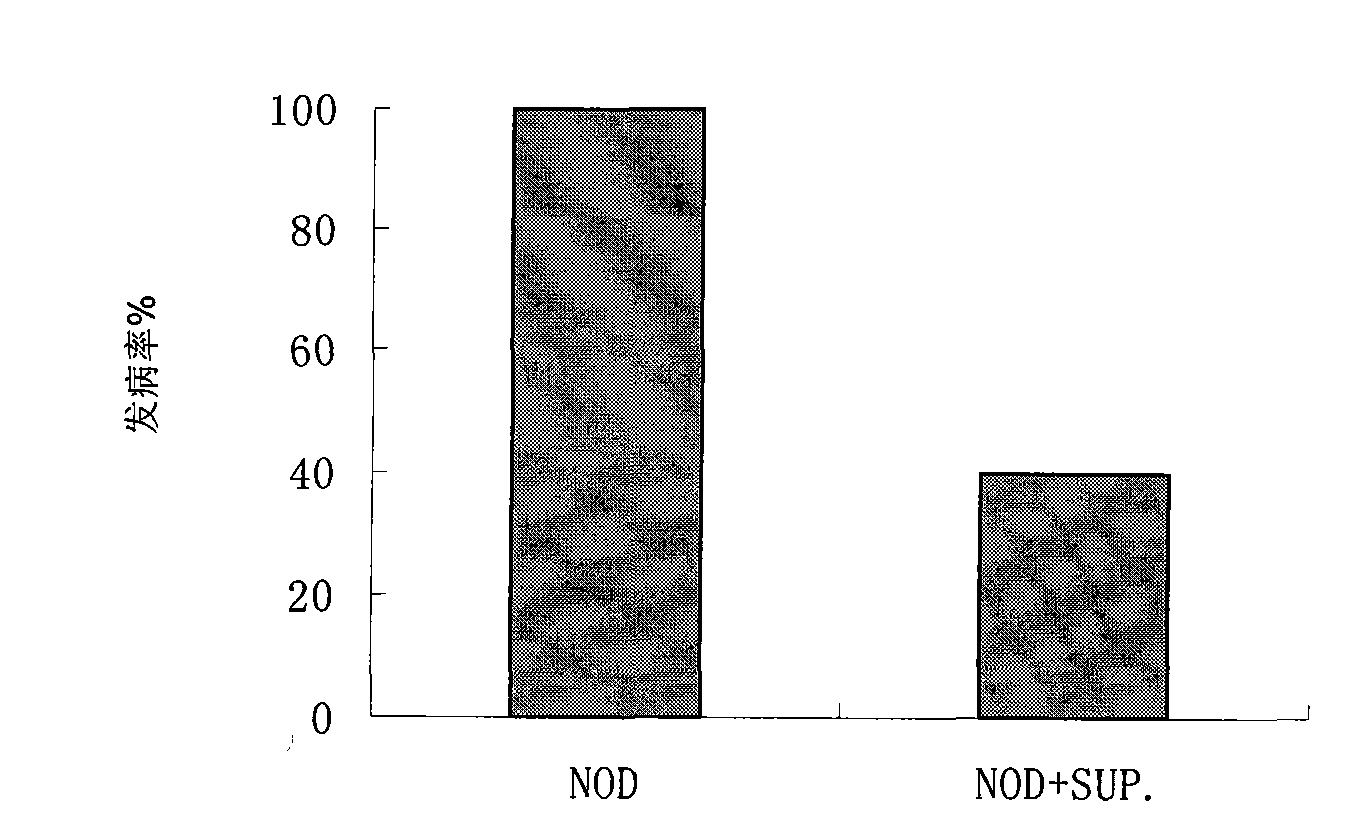
[0069] Result: 100% disease, see figure 1 .
[0070] 2) SUP-NKT control group:
[0071] Inject 2 × 10 to 3-5 week old female NOD mice 9 -5×10 9 / L donor SUP-NKT cells, a total of 2 times. Incidence...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com