Combination and screening method of dominant antigens of Helicobacter pylori based on CD4+ T cell immunization
A technology of Helicobacter pylori and dominant antigen, applied in the field of medicine and biology, can solve the problem that a new type of Helicobacter pylori vaccine has not been developed, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Evaluation of CD4+ T cell response in mice immunized with whole bacteria of H.pylori
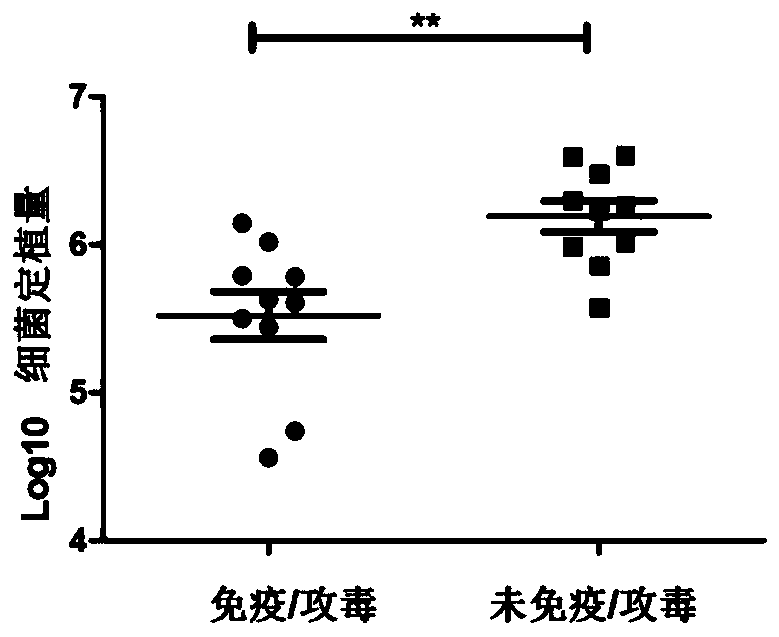
[0056] 1. Experimental protocol for animal immunization and challenge
[0057] Experimental animals: BALB / c female mice aged 6-8 weeks.
[0058] antigen:
[0059] (1) Immune / challenge group (I / C): H.pylori inactivated whole bacteria. 100μg / piece.
[0060] (2) Unimmunized / challenged group (U / C): phosphate buffered saline (PBS).
[0061] Adjuvant: Freund's adjuvant. 100μl / piece.
[0062] Immunization method: subcutaneous injection.
[0063] Immunization volume: 200μl / monkey.
[0064] Immunization scheme: subcutaneous immunization 3 times (week 0, 2, 4). Complete Freund's adjuvant was used for the first time, incomplete Freund's adjuvant was used for the second time, and no adjuvant was added for the third time.
[0065] One week after the last immunization, 1.0×10 9 CFU Helicobacter pylori orally, once a day, for 4 consecutive days. Mice were sacrificed at 4 weeks after chall...
Embodiment 2
[0083] Molecular Sieve Chromatography Divides H.pylori Whole Bacterial Antigens into Different Fractions According to Molecular Size
[0084] First, Helicobacter pylori strain B0 was dissolved in 8mol / L urea, containing 10mmol / L dithiothreitol (DTT) 1g / 6ml, and stirred gently at 4°C for 18 hours. Next, centrifuge at 12,000 g, collect the supernatant, and filter with a 0.2 μM filter. Again, through molecular sieve chromatography, proteins with different molecular weights were divided into 30 groups, and the mixed protein components were named PC01-PC30 ( figure 2 ). Molecular screening uses a 10 / 300GL Superdex 200 chromatographic column, the loading volume is 1ml, the flow rate is set at 0.5mL / min, and 1ml / tube is collected. The obtained protein components were analyzed by sodium dodecyl sulfate polyacrylamide gel (SDS-PAGE) electrophoresis, and the concentration of each protein component was determined by a bicinchoninic acid (BCA) detection kit. see results figure 2 . ...
Embodiment 3
[0086] 3 H-TdR incorporation method to detect the degree of proliferation of CD4+ T cells stimulated by different components
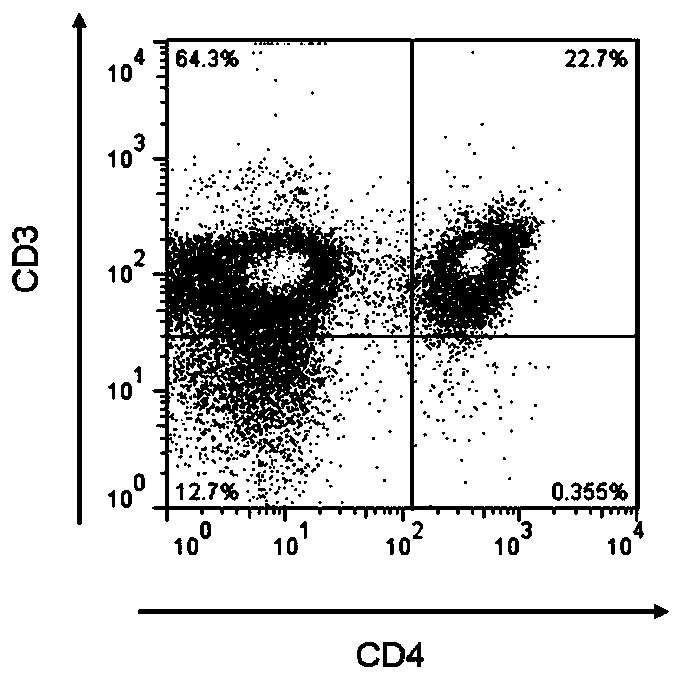
[0087] BALB / c mouse peritoneal macrophages were used as antigen presenting cells (APC). Add 1×10 per well in a 96-well round bottom plate 5 APCs and 200 μL complete RPMI1640 medium. Combine it with H. pylori strain B0 whole antigen or each protein component PC01~PC30 at 37°C 5% CO 2 Co-cultivate in an incubator, the final concentration of each antigen is 50 μg / mL, and three replicate wells are set for each group. Ten hours later, spleen CD4+ T lymphocytes were sorted with magnetic beads from H. pylori-immunized / infected and H. pylori-immunized / uninfected mice 4 weeks later. 1×10 5 Splenic CD4+ T lymphocytes were co-cultured with the above APCs for 96 hours. joined in the last 18 hours 3 H-TdR 1 μCi / well. Afterwards, the CPM value of each well was detected with a liquid scintillation counter, and expressed as a stimulation index (SI), and SI was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com