Recombination interferon with new space conformation and enhanced effect, its preparing method and application
A recombinant interferon and sequence technology, which is applied to the composition of the same substance, the pharmaceutical composition, and the application field of medicine, can solve the problems of inability to use large doses, large side effects, and inability to directly inhibit the secretion of HBsAg and HBeAg, and achieves Reduce toxic side effects, low side effects, and inhibit secretion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
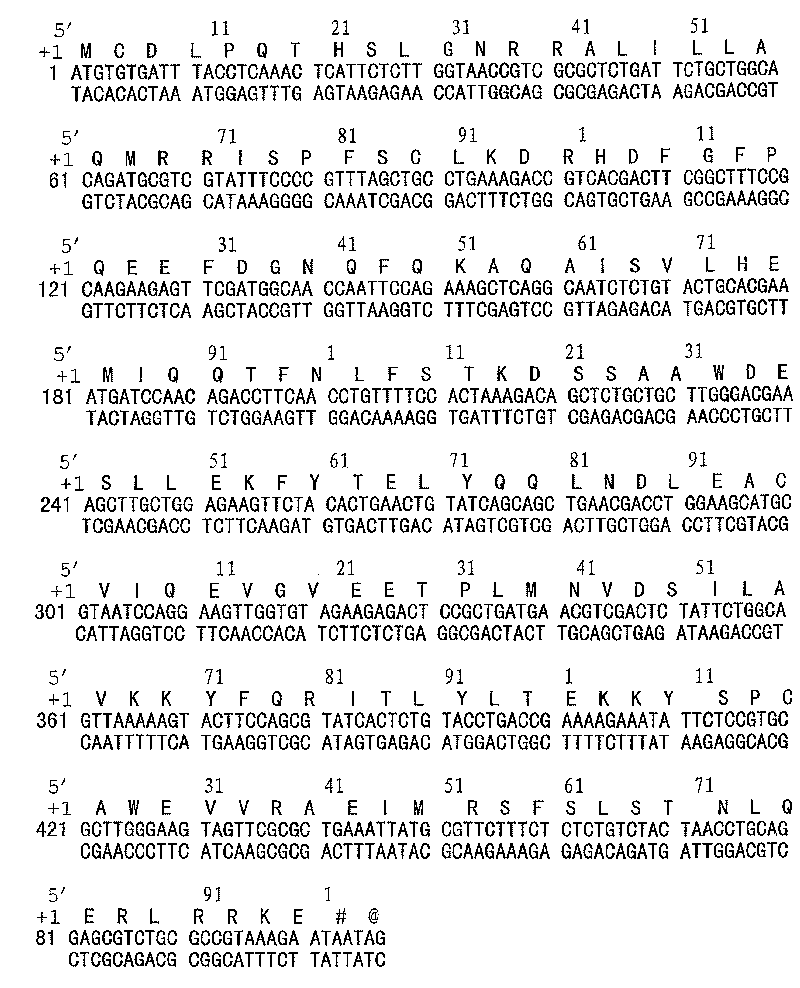
[0076] According to the published DNA sequence encoding interferon alfacon-1 and deduced amino acid sequence data (Klein ML, Bartley TD, Lai PH, et al., Structural characterization of recombinant consensus interferon-alpha. Journal of Chromatography, 1988; 454:205-215), see figure 1 , we used Escherichia coli to preferentially express codons (The Wisconsin Package, by Genetics Computer Group, Inc. Copyright 1992, Medison, Wisconsin, USA), and under the condition that the amino acid sequence remained unchanged, molecular design was carried out for its DNA coding sequence, Then the full-length cDNA encoding gene of rSIFN-co was artificially synthesized.
[0077] In order to obtain a large amount of high-purity rSIFN-co protein for research and clinical treatment, we used recombinant DNA technology to clone the full-length cDNA sequence of rSIFN-co into the high-efficiency expression vector of E. coli, and then induced / activated the expression with L-arabinose The regulatory me...
example 2
[0136] Isolation and Purification of Recombinant Interferon (rSIFN-co)
[0137] Fermentation of engineered bacteria
[0138] Inoculate the engineered bacteria in LB medium, 37 ° C, shake the flask (200rpm) and cultivate overnight (about 18 hours), add 50% glycerol with a concentration of 30% to the bacterial culture solution, mix and divide into 1ml each, Store at -20°C as a production strain.
[0139] The production strain was inserted into LB medium (containing 10 g of peptone, 5 g of yeast extract, and 10 g of sodium chloride in 1 L) at a ratio of 1%, cultivated overnight at 37° C. at 200 rpm, and was used as a grade I seed fungus. Grade I seed bacteria are added into RM medium in a ratio of 10% (1L contains 20g of casein, 1mmol / L of magnesium chloride (0.203g), 4g of disodium hydrogen phosphate, 3g of potassium dihydrogen phosphate, 0.5g of sodium chloride, chlorine amine 1g), 37°C, pH 7.0, fermented to OD 600 Add arabinose (20%) to a final concentration of 0.02% to ind...
example 3
[0154] Differences in spatial conformation between recombinant interferon (rSIFN-co) of the present invention and interferon alfacon-1
[0155] The spatial conformation difference between the recombinant interferon (rSIFN-co) of the present invention and interferonalfacon-1 was compared by circular dichroism method.
[0156] Adopt circular dichroism instrument J-500C, set measurement sensitivity to be 2m° / cm, optical path be 0.20cm, measure the circular dichroism spectrum of dried rejuvenated sample in wavelength range 190nm-250nm; Described dried rejuvenated sample contains 30μg / ml Dry Fujin, 5.9mg / ml NaCl, 3.8mg / ml Na 2 PO 4 , pH 7.0. The result is shown in Fig. 6A.
[0157] Adopt circular dichroism instrument J-500C, setting and measuring sensitivity is 2m ° / cm, and optical path is 2cm, and measuring recombinant interferon (rSIFN-co) sample of the present invention is the circular dichroism spectrum in the wavelength range of 250nm-320nm; The above interferon samples co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com