Preparation method of multidimensional multivalence prostatic cancer specificity tumor antigen assembly
A technology for prostate cancer and tumor antigen, applied in the field of biomedicine preparation, can solve the problems of low immune killing and poor treatment effect, and achieve the effect of long stimulation time, inhibiting and killing prostate cancer, and good effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
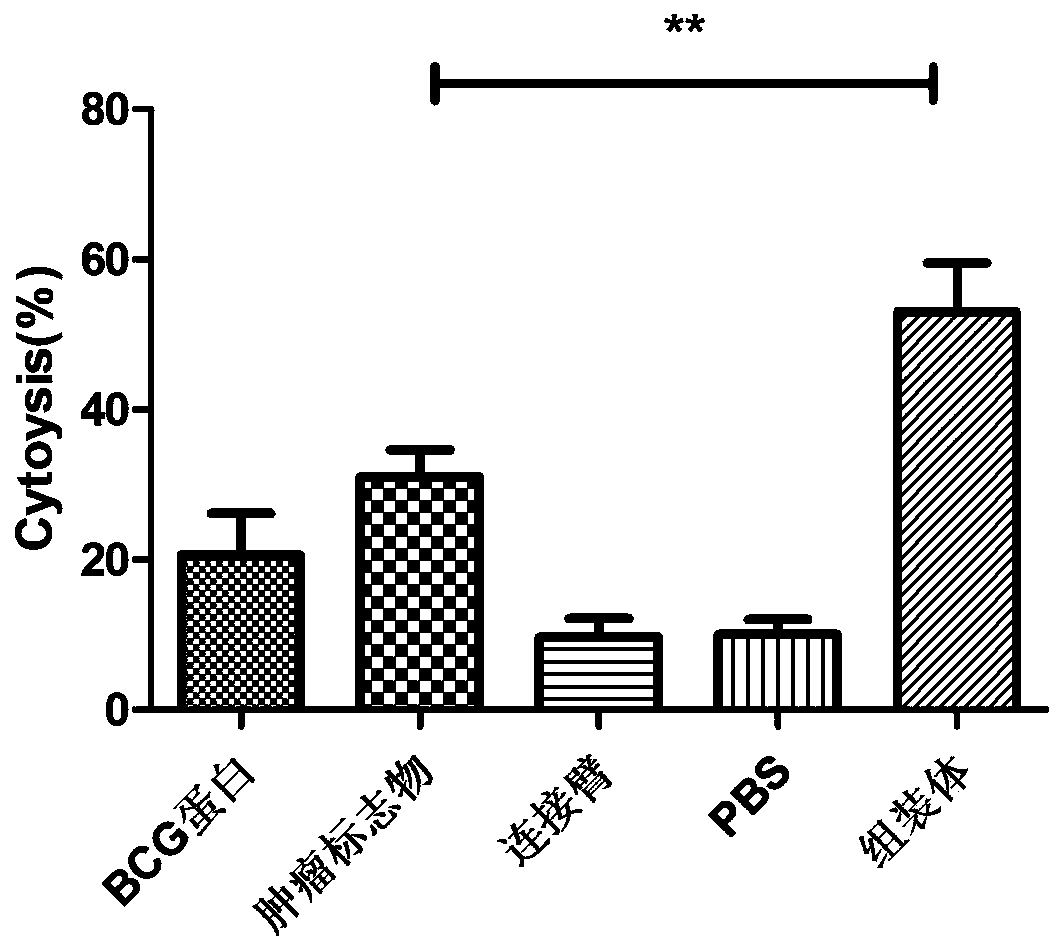
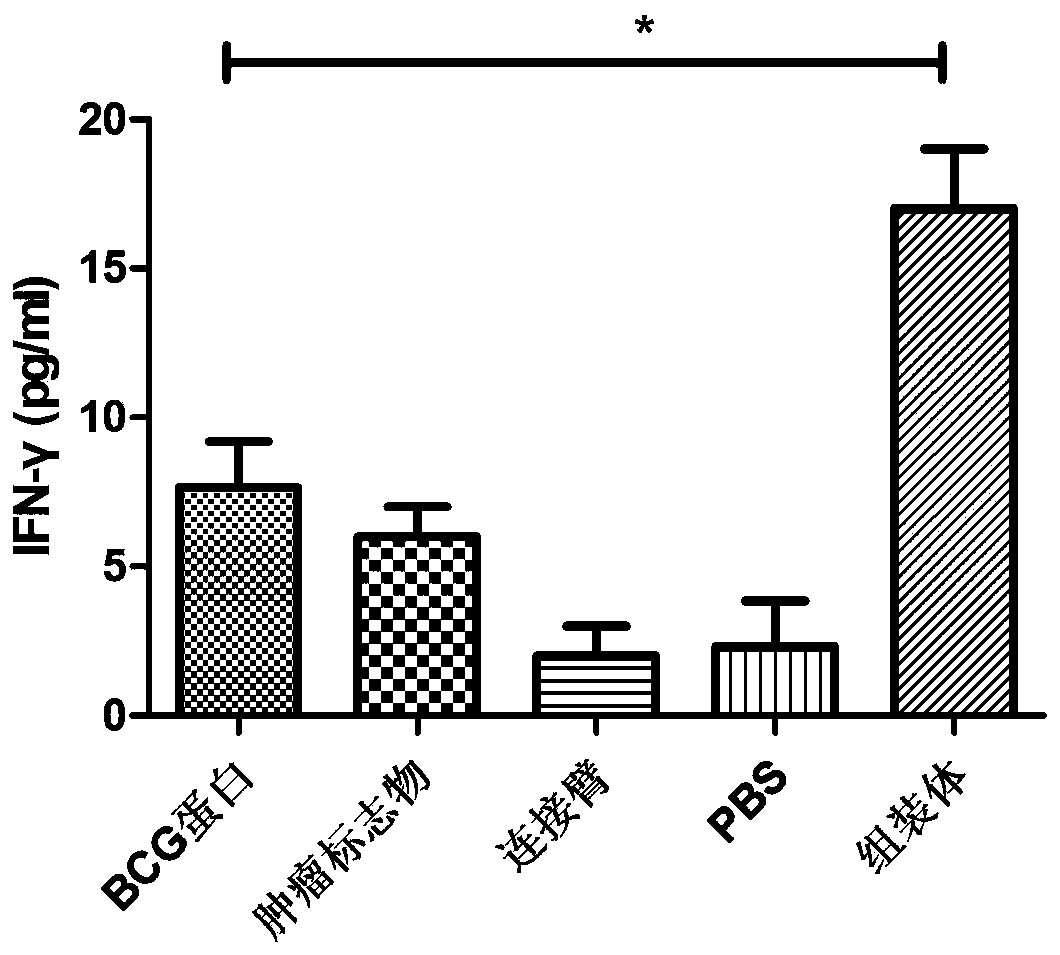
Image
Examples
Embodiment 1
[0024] 1. Materials and methods
[0025] 1.1.1 Strains: The strains used are derived from Mycobacterium bovis (BCG, BCG; Pasteur strain) provided by ATCC (authorized by ATCC and signed a development and use agreement).
[0026] 1.1.2 Culture medium: purchased from sigma company.
[0027] 1.1.3 Nano-polymer materials:
[0028] Chitosan, polyglutamic acid, etc.; purchased from Tianjin Zhongao Tianyuan Technology Development Co., Ltd.
[0029] 1.1.4 General chemical reagents: (1) EDS (ethylenediaminetetraacetic acid), NHSS (N-hydroxysulfosuccinic acid amine); (2) sulfate; (3) organic acid; (4) inorganic salt, etc.;
[0030] All were purchased from Tianjin Sino-Australian Tianyuan Technology Development Co., Ltd.
[0031] 1.1.5 Prostate cancer tumor markers PSMA, PAP, STEAP1, and ACPP protein coding sequences were synthesized by Tianjin "Jinweizhi" company with reference to the standards published by "NCBI".
[0032] 1.1.6 Cloning Pichia pastoris strains and plasmids were used...
Embodiment 2
[0044]1. Modification and synthesis of graphene oxide (GO) modified polypeptide:
[0045] Take 10 mL of an aqueous solution made of nanographene oxide (GO), (1 mg / mL), add EDS (5 mg) and NHSS (4 mg) into the GO aqueous solution, and stir for 30 min. Then 3 mL (1 mg / mL) of each (PSMA, PSCA, PAP or STEAP) polypeptide aqueous solution was added, and the reaction was stirred at room temperature for 48 h. The reaction solution was centrifuged, the supernatant was discarded, the obtained precipitate was washed with water three times, and the precipitate was collected by centrifugation. It was stirred in a weakly acidic aqueous solution for 72 hours at a low speed, and re-dispersed in 10 mL of deionized water to obtain a graphene oxide (GO) modified polypeptide stock solution. and stored at 4°C. 2. Graphene oxide (GO) - protein connection modification:
[0046] Take 2 mL of graphene oxide (GO) modified polypeptide stock solution, add it to the separated and purified BCG bacterial ...
Embodiment 3
[0048] 1. Polyglutamic acid-polypeptide modification solution.
[0049] Dilute each (PSMA, PSCA, PAP or STEAP) polypeptide stock solution (1mg / mL) with PBS solution, add to polyglutamic acid containing 5% acetic acid dispersion solution (1mg / mL), the ratio is set at 3:1~ 6:1, vortex, mix thoroughly, place at 4°C, stir at low speed, overnight. The prepared polyglutamic acid-polypeptide solution was obtained.
[0050] 2. Polyglutamic acid polypeptide-protein connection modification:
[0051] Take 2 mL of the prepared polyglutamic acid-polypeptide liquid peptide stock solution, add it to the separated and purified BCG bacterial protein PBS solution (containing 2 mg / 8 mL), and adjust the pH to neutral. Add EDS and NHSS and stir for 30 minutes to fully dissolve. Adjust the pH to 7.0, stir at a low speed at 20°C, and react with stirring for 24 hours. After the reaction, the "assembly" solution was obtained, which was stored at 4°C for future use.
[0052] 1.4. Electrophoretic m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com