Monoclonal antibody for resisting GPC3
A monoclonal antibody, cgmccno.3232 technology, applied in the field of biomedicine, can solve the problems of limited specificity and sensitivity, few types of hepatocyte-specific antibodies, inconvenient pathological diagnosis and differential diagnosis of liver cancer, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Expression of Human GPC3 Protein Fragment
[0052] 1.1 Amplification of GPC3-N-terminal gene fragment
[0053] A 981bp GPC3-N-terminal gene fragment was cloned from human placenta cDNA by PCR method as an amplification template.
[0054] Design the following PCR primers:
[0055] Primer 1:
[0056] 5’-CGGC GAA TTC AA GCC ACC TGT CAC CAA GTC-3’
[0057] Primer 2:
[0058] 5’-CGC CTC GAG TCA AAA TCT ATA TTG GCG TTG-3’
[0059] PCR reaction system:
[0060] KOD enzyme, 1 μl; KOD buffer night, 5 μl; 25mmol / L MgCl 2 , 3 μl; 10 μmol / L P1, 1 μl; 10 μmol / L P2, 1 μl; dNTP, 5 μl; template, 0.5 μl; ddH 2 O, 34.5 μl; a total of 50 μl.
[0061] Pre-denaturation at 94°C for 4 minutes; then denaturation at 94°C for 30 sec, annealing at 57°C for 30 sec, chain extension at 68°C for 60 sec, 30 cycles.
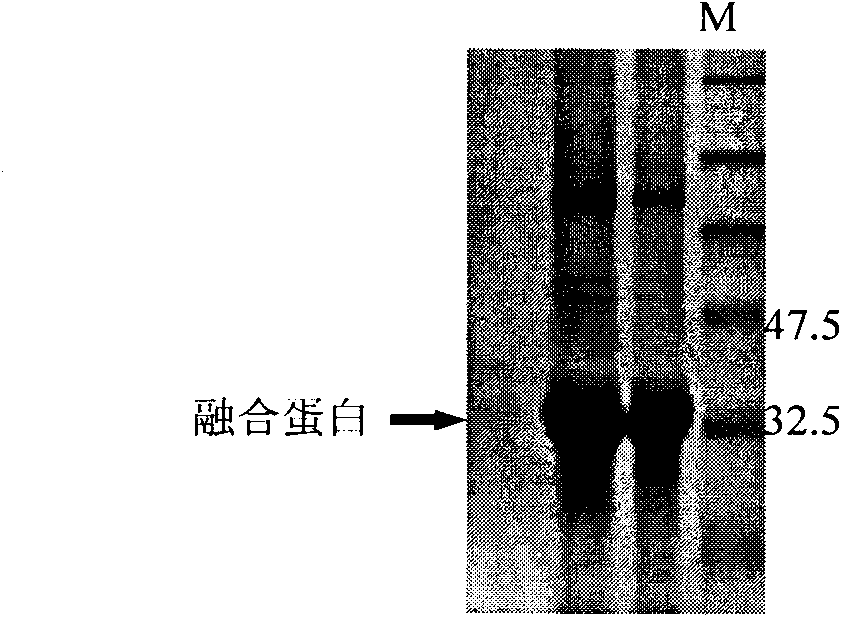
[0062] The resulting PCR product was purified with a gel extraction kit (Qiagen Gel Extraction Kit). With reference to the method described in "Molecular Cloning" J. Sambrook,...
Embodiment 2
[0071] Preparation and purification of Z1C15 monoclonal antibody
[0072] 2.1 Immunization of mice with GPC3 fusion protein
[0073] Mix the GPC3 protein purified in Example 1 with complete Fred's adjuvant (CFA) and immunize 5-6 week-old female Bab / c mice (Experimental Animal Center of Second Military Medical University), intradermal multipoint injection, 100 μg / Only. After 4 weeks, the first booster immunization was injected intraperitoneally, 100 μg per mouse. After 6 weeks, the second booster immunization was injected intraperitoneally, 100 μg per mouse. At the 8th week of immunization, the tail blood of the immunized mice was taken to measure the titer, and the GPC3 antigen was applied to coat with 3 μg / ml, and 10% FCS was blocked. color, using a microplate reader (Bio-RAD 550) at OD 492 down reading.
[0074] 2.2 Establishment and screening of hybridoma cell lines secreting anti-GPC3 protein antibody
[0075] Mouse myeloma SP2 / 0 cells were prepared at the same ...
Embodiment 3
[0083] Identification of Z1C15 mAb
[0084] 3.1 In vitro culture of Z1C15 cells
[0085] Use the Z1C15 hybridoma cell CGMCC No.3232 prepared in Example 2 to carry out in vitro cell culture (DMEM, 10% FBS medium, 37 ° C, 5% CO 2 cultured in an incubator) to obtain a large amount of hybridoma cell supernatant.
[0086] 3.2 Type identification of monoclonal antibodies
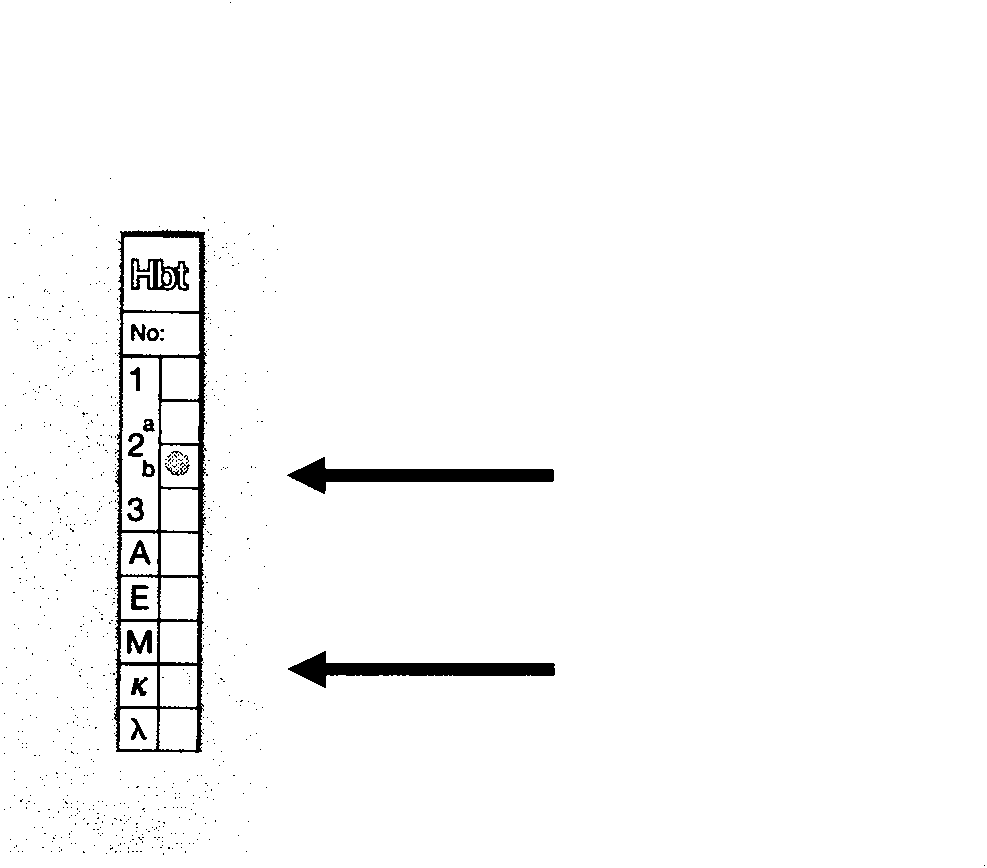
[0087] Mouse Monoclonal Antibody Ig Class and Subclass Detection Kit (HyCult biotechnology bv MouseMonoclonal Antibody Isotyping Kit, P / N: HL2010) was used to identify Ig class and subclass. figure 2 . Studies have shown that the monoclonal antibody heavy chain is IgG2b type, and the light chain is κ chain.
[0088] 3.3 Affinity determination of monoclonal antibody to GPC3 protein
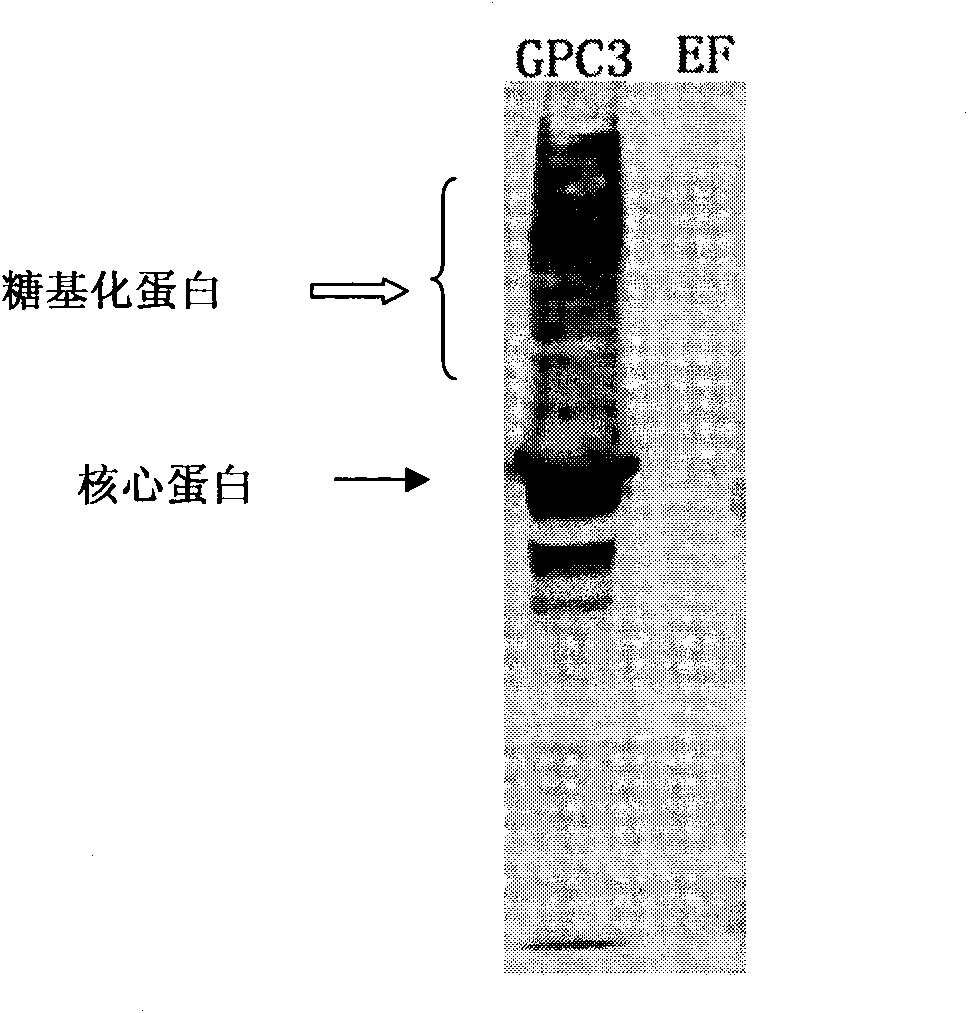
[0089] To identify whether the monoclonal antibody can recognize the exogenous GPC3 protein. With reference to the method described in "Molecular Cloning" J. Sambrook, D.W. Russell (U.S.), translated by Huang Peitang, etc., ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com