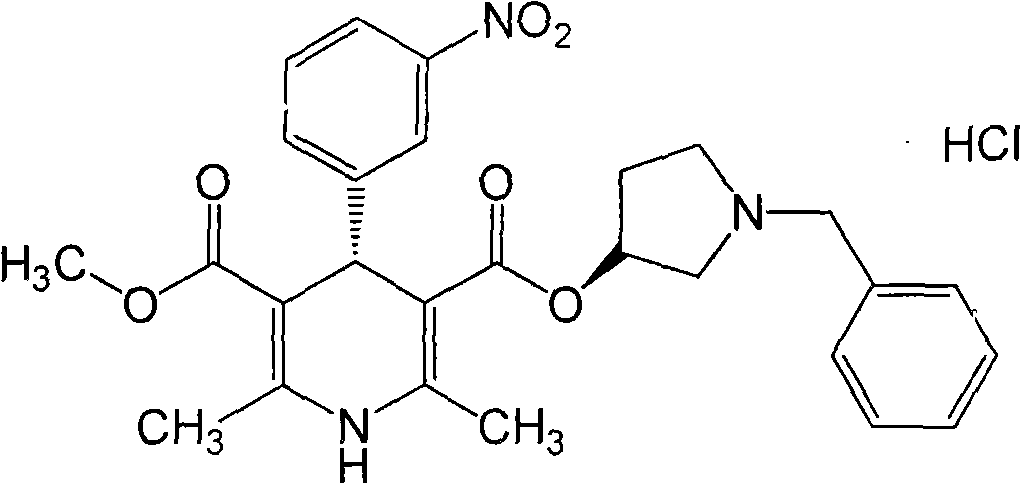
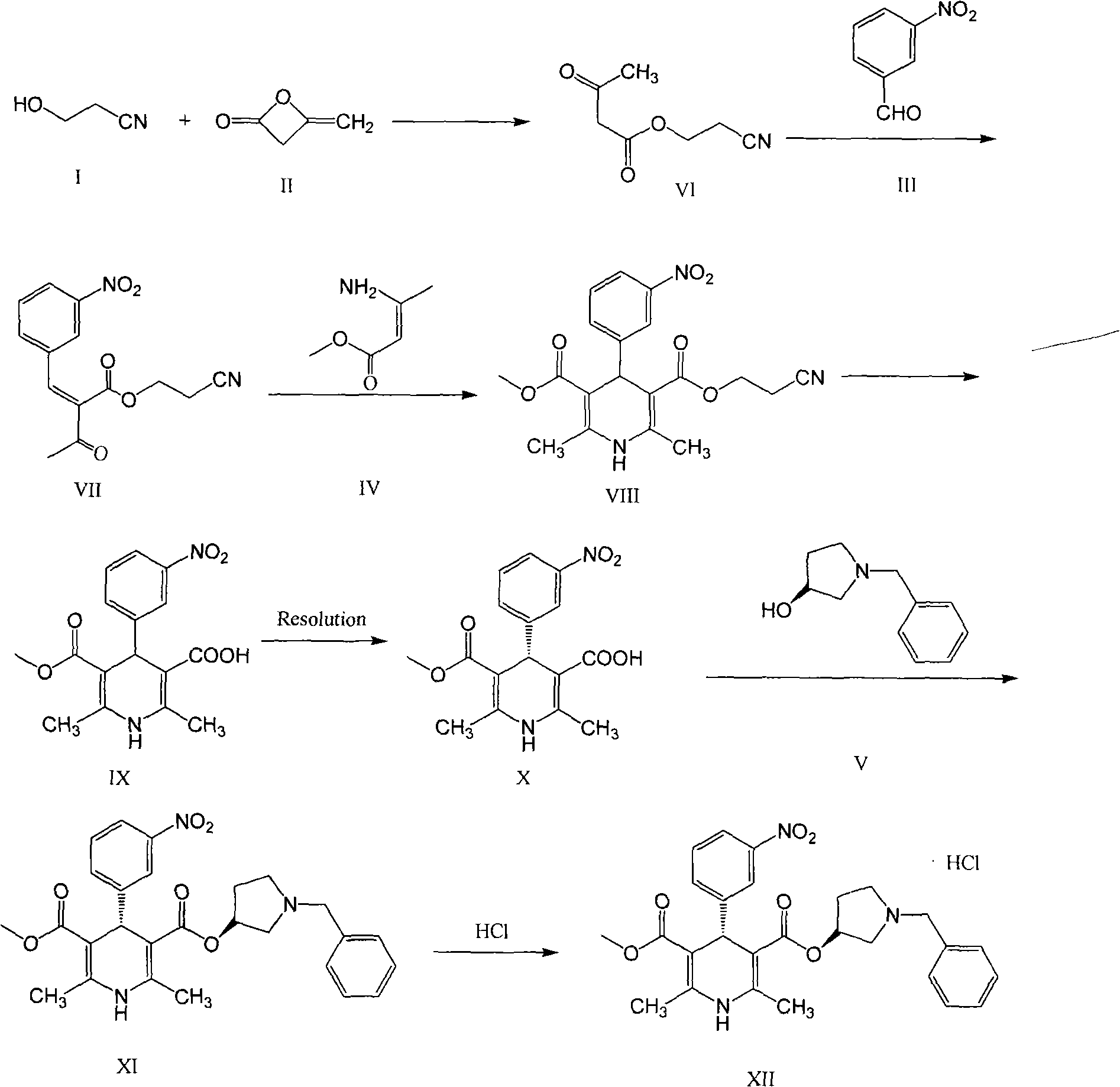
Synthesis process of barnidipine hydrochloride
A technology of barnidipine hydrochloride and synthesis process, which is applied in the fields of drug combination, cardiovascular system diseases, organic chemistry, etc. low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0035] The specific implementation steps are as follows:
[0036] 1. In a dry 500ml single-necked flask, add 3-hydroxypropionitrile (117g, 1.648mol) and 4g of triethylamine, raise the temperature to 80°C, then slowly add diketene (159.0g, 1.892mol) dropwise. After stirring for more than 6 hours, the low boiling point substances were evaporated to obtain 137 g of a reddish-brown oily substance, which was compound (VI), with a yield of 54%.
[0037] 2. In a dry 2000ml single-necked flask, add m-nitrobenzaldehyde 67.5g (0.447mol), compound (VI) 69.3g (0.447mol), ammonium acetate 5g, isopropanol 318g, and stir at room temperature for 15 hours , a large amount of white solid was formed in the reaction. After the reaction was completed, 104 g of white solid was obtained by filtration, which was compound (VII), and the yield was 80.8%.
[0038] 3. 74.9 g (0.26 mol) of compound (VII), 30 g (0.26 mol) of methyl β-aminocrotonate and 180 ml of methanol were refluxed for 2 hours. After...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com