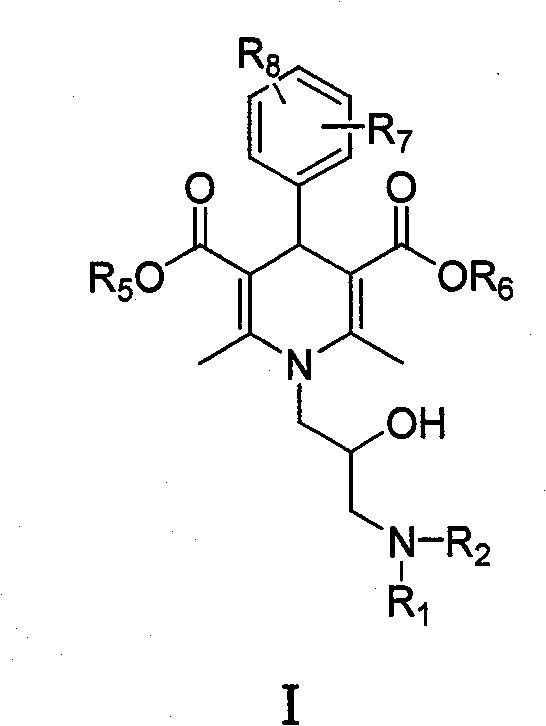
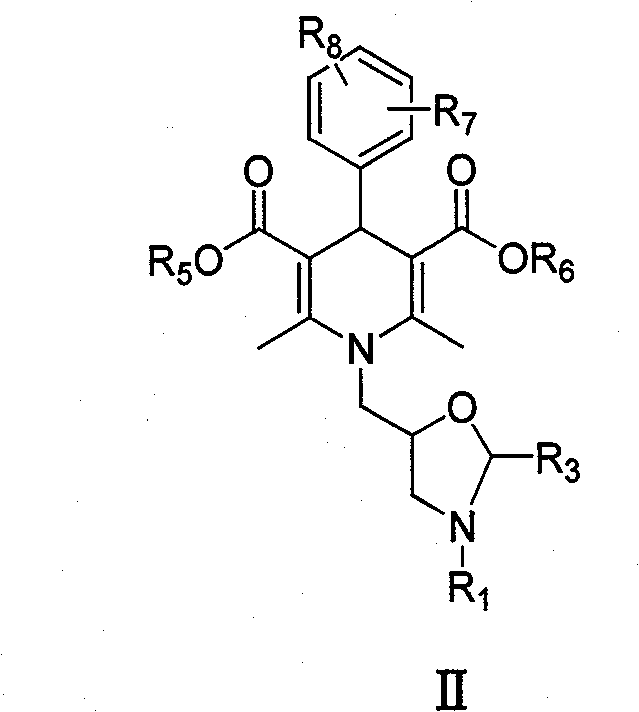
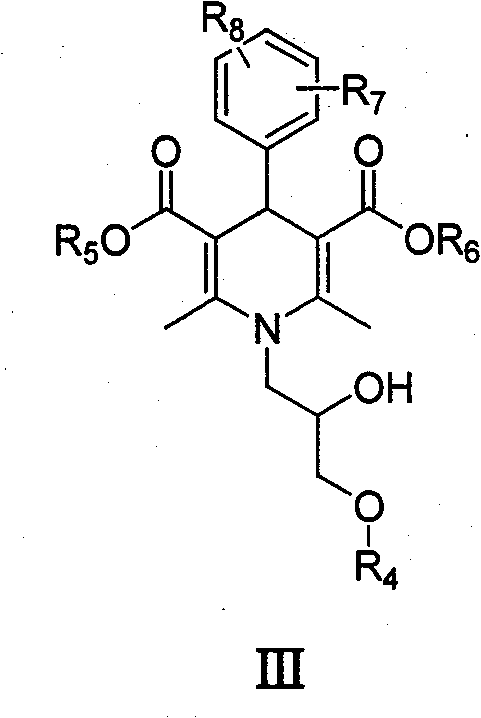
Dihydropyridine type tumor chemotherapeutic sensitizer and application thereof
A dihydropyridine and compound technology, applied in the field of dihydropyridine tumor chemotherapy sensitizers, can solve the side effects of cardiovascular system and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Example 1: 2,6-Dimethyl-4-(3-nitrophenyl)-1-[(oxiranyl)methyl]-1,4-dihydropyridine-3,5-dicarboxy Acid dimethyl ester (intermediate)
[0064] Add 2.31g (57.8mmol) 60% sodium hydrogen and 50mL anhydrous tetrahydrofuran to the reaction flask, stir, and add 10g (28.9mmol) 2,6-dimethyl-4-(3-nitrophenyl) at 0°C -Dimethyl 1,4-dihydropyridine-3,5-dicarboxylate, after no gas is released, add 7.98g (86.7mol) of epichlorohydrin dropwise. After the addition, continue to stir for 0.5h, and react at room temperature for 24h. Slowly add 5 mL of water dropwise, concentrate under reduced pressure, add 20 mL of dichloromethane and 10 mL of water to the residue, stir for 10 min, separate the layers, extract the aqueous layer with 2×5 mL of dichloromethane, combine the organic layers, dry over anhydrous sodium sulfate, and filter. Concentration under reduced pressure, the residue was separated by column chromatography (petroleum ether: ethyl acetate = 3:2) to obtain 5.30 g of light yello...
Embodiment 2
[0065] Example 2: 1-[3-(2-hydroxyethylamino)-2-hydroxypropyl]-2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydro Dimethyl pyridine-3,5-dicarboxylate (I 1 )
[0066] Add 0.5g (1.25mmol) 2,6-dimethyl-4-(3-nitrophenyl)-1-[(oxiranyl)methyl]-1,4-dihydropyridine to the reaction flask -Dimethyl 3,5-dicarboxylate, 0.23g (3.75mmol) ethanolamine and 10mL methanol, stirred and refluxed for 5h, concentrated under reduced pressure, and the residue was column chromatographed (petroleum ether: ethyl acetate: triethylamine=30: 6:1) to obtain 500 mg of pale yellow oil, with a yield of 86.8%.
[0067] 1 HNMR (CDCl 3 )δ: 2.43~2.74(m, 4H, CHC H 2 NHC H 2 CH 2 OH), 2.50, 2.55 (2×s, 6H, C 2,6 -CH 3 ), 3.65(t, J=5.1Hz, 2H, CH 2 C H 2 OH), 3.69~3.74 (m, 1H, C H OH), 3.71, 3.73 (2×s, 6H, C 3,5 -COOCH 3 ), 3.84 (m, 2H, NC H 2 CH), 5.21(s, 1H, C 4 -H), 7.39(m, 1H, nitrogenyl 5-H), 7.69(m, 1H, nitrogenyl 6-H), 7.98~8.00(m, 1H, nitrogenyl 4-H), 8.15(m, 1H, nitrogenyl 2 -H)
[0068] ESI-MS(m / ...
Embodiment 3
[0071] Example 3: 1-[(2-Hydroxy-3-n-butylamino)propyl]-2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine- 3,5-dicarboxylic acid dimethyl ester (I 2 )
[0072] Refer to I 1 Synthetic method, a light yellow oily substance was obtained with a yield of 90.3%.
[0073] 1 HNMR (CDCl 3 )δ: 0.90 (t, J=7.1Hz, 3H, CH 2 CH 2 CH 2 C H 3 ), 1.30~1.43 (m, 4H, CH 2 C H 2 C H 2 CH 3 ), 2.27~2.55(m, 4H, CHCH 2 NHC H 2 CH 2 CH 2 CH 3 ), 2.49, 2.55 (2×s, 6H, C 2,6 -CH 3 ), 3.57~3.62 (m, 1H, C H OH), 3.71, 3.72 (2×s, 6H, C 3,5 -COOCH 3 ), 3.82 (d, J=5.4Hz, 2H, NC H 2 CH), 5.21(s, 1H, C 4 -H), 7.37(m, 1H, nitrogenyl 5-H), 7.70(m, 1H, nitrogenyl 6-H), 7.97~8.00(m, 1H, nitrogenyl 4-H), 8.15(m, 1H, nitrogenyl 2 -H)
[0074] ESI-MS(m / z): 476[M+H] +
[0075] IR: 3400, 3032, 2948, 1692, 1636, 1577, 1571, 1527, 1430, 1386, 1349, 1203, 1157, 772
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com