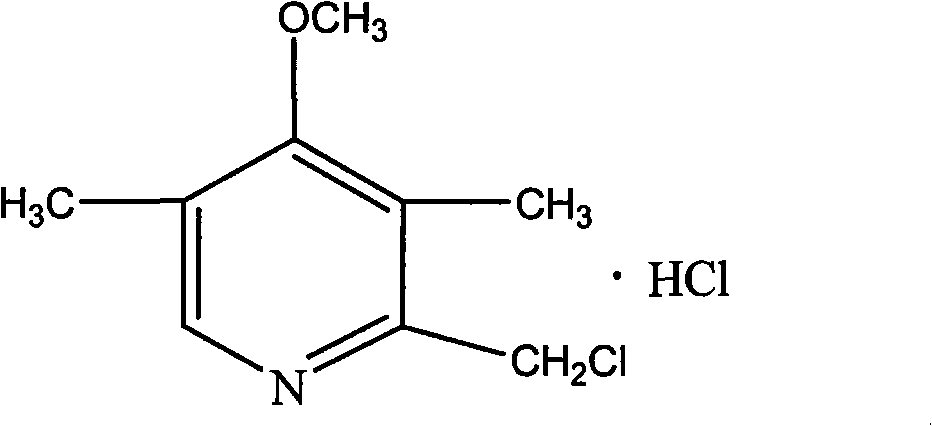
Purifying method of 2-chloromethyl-4-methoxyl-3,5-dimethylpyridine chloride
A technology of lutidine hydrochloride and lutidine, which is applied in the chemical field, can solve the problems of high solvent cost, influence on yield, hidden safety hazards, etc., and achieve the effects of cost reduction, simple operation, and favorable industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: 2-chloromethyl-4-methoxy-3,5 obtained by using 3,5-lutidine as a raw material through oxidation, nitration, methoxylation, acetylation, hydrolysis, and chlorination steps -The lutidine hydrochloride reaction liquid obtained 100g of brown solid after removing the solvent, and washed with a mixed solvent with a volume ratio of acetone:petroleum ether=2:1, and washed 2 to 3 times, with an amount of 150ml / time. After filtering and drying in an oven at 50° C. for 1 hour, 85 g of white crystal 2-chloromethyl-4-methoxy-3,5-lutidine hydrochloride was obtained, and the purity analysis by HPLC was 99.54%.
Embodiment 2
[0022] Example 2: Using 2,3,5-collidine as raw material, 2-chloromethyl-4-methoxy- The 3,5-lutidine hydrochloride reaction solution was desolvated to obtain 100 g of a brown solid, which was washed with a mixed solvent of acetone:petroleum ether=2.5:1, and washed 2 to 3 times with a dosage of 200ml / time; After drying in an oven at 80° C. for 2 hours, 83.6 g of white crystal 2-chloromethyl-4-methoxy-3,5-lutidine hydrochloride was obtained, and the purity analysis by HPLC was: 99.49%.
Embodiment 3
[0023] Example 3: Using 3,5-lutidine as a raw material to obtain 100 g of solids through oxidation, nitration, methoxylation, acetylation, hydrolysis, and chlorination steps, the volume ratio is acetone:petroleum ether=3:1 Wash with a mixed solvent, wash 2 to 3 times, the dosage is 200ml / time; filter and oven dry for 1 hour to obtain 82.01g of white crystal 2-chloromethyl-4-methoxy-3,5-dimethyl Pyridine hydrochloride, HPLC purity analysis: 99.51%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com