Recombinant anthropogenic hepatocyte growth factor (HGF) activating factor and application thereof
An activator, human-derived technology, applied in applications, genetic engineering, plant genetic improvement, etc., can solve the problems of lack of treatment methods and high mortality of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: Preparation of recombinant human HGFA-Fc fusion protein
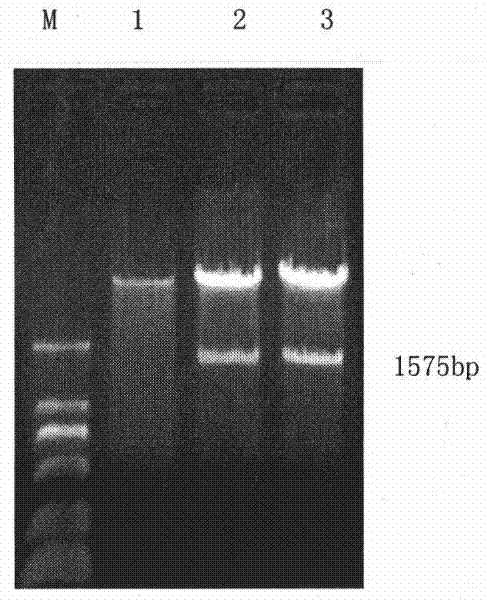
[0034] Use RT-PCR to catch the HGFA active region DNA fragment (GeneID: 3083, 1104bp ~ 1962bp) from the liver tissue cDNA library with a size of 858bp, and use Overlap overlapping PCR (see Cao Yang et al., "Hebei Medicine", Volume 27, Issue 11, 2005) Fusion with human immunoglobulin Fc (GeneID: 2209) 693bp fragment to obtain 1575bp HGFA-Fc fragment, Xho I / Hind III double digestion and cloning into pcDNA3.1(+) vector. After the enzyme digestion and identification are correct, a large amount of extraction, purification, and concentration determination are used for later use. Specific steps are as follows:
[0035] 1. Construction of recombinant plasmid pcDNA3.1-HGFA-Fc:
[0036] 1. Primer synthesis for amplifying the HGFA-Fc chimeric gene
[0037] The human HGFA gene (ID: 3083) was determined according to the gene library, and primers were designed to amplify the range of 1102bp to 1965bp of the human...
Embodiment 2
[0070] Example 2: Enzyme cleavage activity and anti-apoptosis effect of rhHGFA-Fc recombinant fusion protein
[0071] 1. Detection of pro-HGF enzyme activity activated by HGFA-Fc:
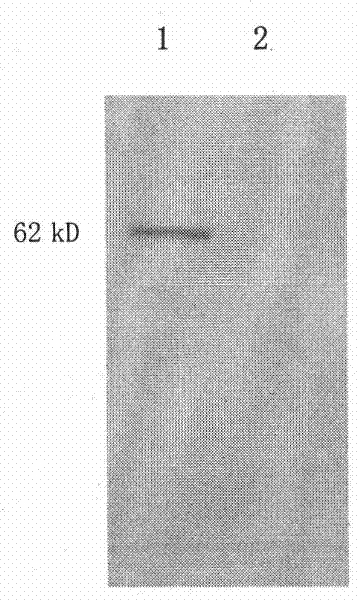
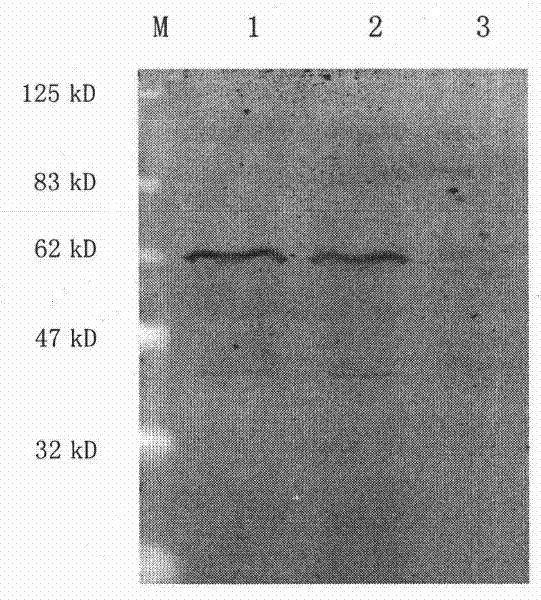
[0072] In the pro-HGF reaction buffer with a final concentration of 50ug / ml, add HGFA-Fc concentration gradients of 0.15ug / ml, 0.3ug / ml, 0.6ug / ml, 0.12ug / ml, 0.24ug / ml, 4.8 ug / ml, Western blot was used to detect the expression of proHGF (92kD) and HGF (62kD) in each reaction system after 1 hour of reaction. The results show that rhHGFA-Fc recombinant protein can digest pro-HGF into α chain and β chain, and the enzymatic activity increases with the increase of rhHGFA-Fc concentration. The results are shown in Figure 4 . After calculation, the IC50 enzyme activity value of the recombinant protein is 20nM.
[0073] In the pro-HGF solution with a concentration of 50ug / ml, HGFA-Fc fusion protein with a final concentration of 2.4ug / ml was added, and HAI-1 with a final concentration of 0.5uM, 2uM and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com